Treatment method for analyzing waste liquid
A technology for analyzing waste liquid and treatment methods, which is applied in the treatment of waste liquid containing Cr6+, Cr3+, Fe3+, and in the field of waste liquid produced by COD analysis by potassium dichromate method, which can solve the problem of unsuitable separation of waste liquid, safety and harmlessness, and resources waste and other problems, to achieve the effect of enhancing the curing and stabilization effect, eliminating environmental pollution, and reducing treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
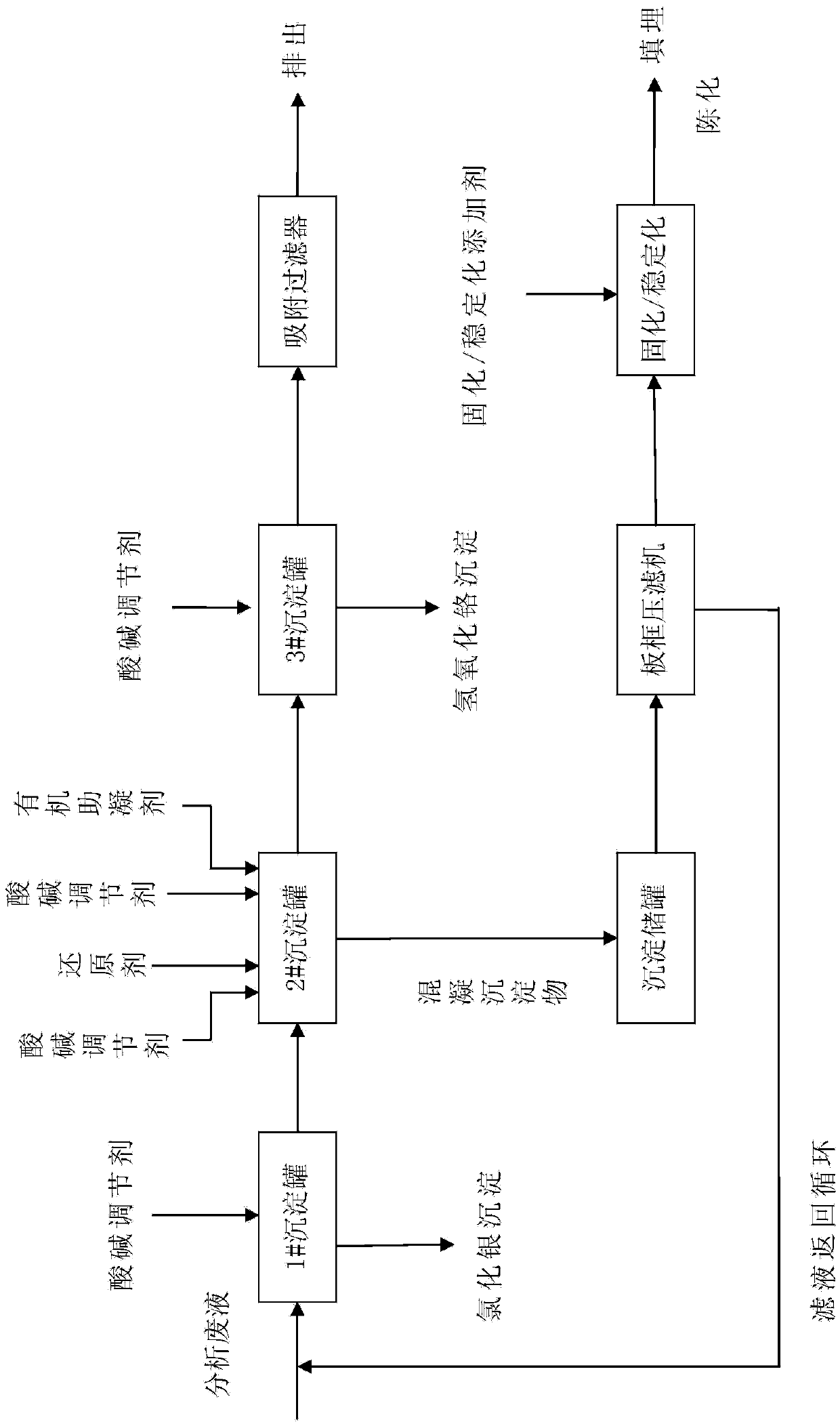
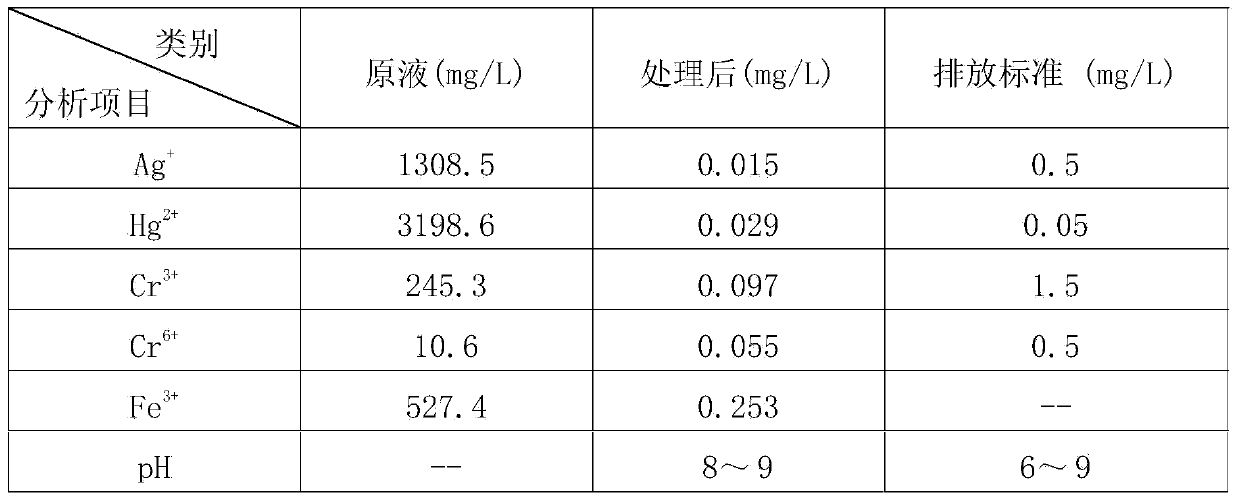
[0045] Please refer to figure 1 Shown is a process flow diagram of a preferred embodiment of the treatment method for analyzing waste liquid of the present invention. Potassium dichromate method measures the analytical waste liquid 1000ml that COD produces and is placed in 1# sedimentation tank, adds sodium chloride 3g in described waste liquid, after suitably stirring, leave standstill, Ag + Converted to silver chloride precipitation, filtration and separation, silver chloride recovery, and the waste liquid is transferred to 2# precipitation tank;
[0046] Use sodium hydroxide solution to adjust the pH value of the waste liquid in the 2# precipitation tank to 2.5, add 25g of ferrous sulfate hexahydrate, preferably stir and react at a speed of 150 rpm for 30min;
[0047] Use sodium hydroxide solution to adjust the pH value of the above waste liquid to 11, and add 10 mg of polyacrylamide, preferably stir and react at a speed of 150 rpm for 2 minutes, then stir and react at a s...
Embodiment 2
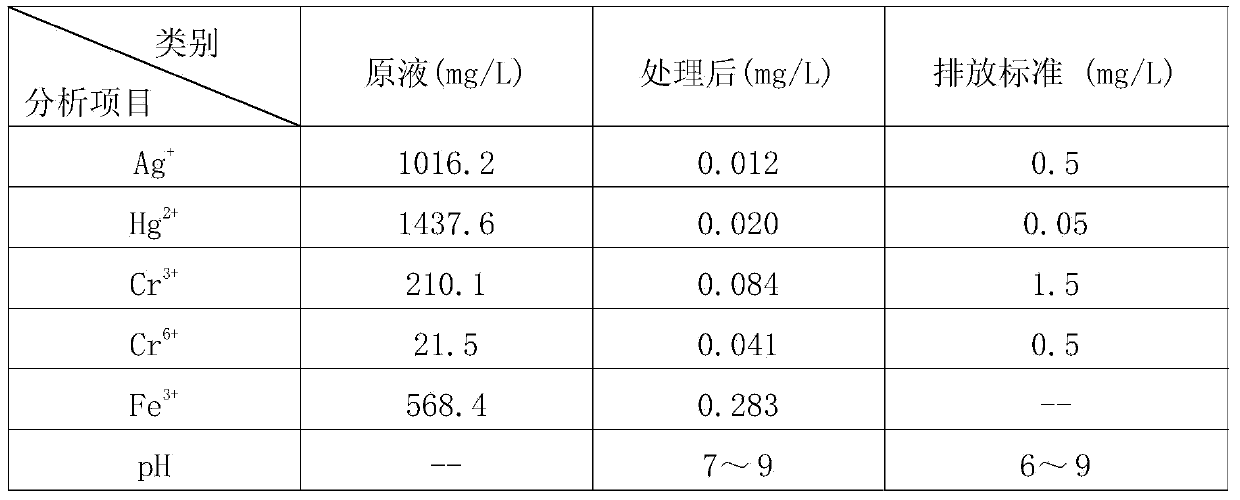
[0054] Please refer to figure 1 Shown is the process flow diagram of the treatment method for analyzing waste liquid of the present invention. Potassium dichromate method measures the analytical waste liquid 1000ml that COD produces and is placed in 1# sedimentation tank, adds sodium chloride 2g, ferrous chloride 1.5g in described waste liquid, after suitably stirring, leave standstill, Ag + Converted to silver chloride precipitation, filtration and separation, silver chloride recovery, and the waste liquid is transferred to 2# precipitation tank;
[0055] Use sodium hydroxide solution to adjust the pH value of the waste liquid in the 2# precipitation tank to 3.0, add 15g ferrous sulfate hexahydrate and 25g sodium sulfite, preferably stir and react at a speed of 150 rpm for 30min;
[0056] Use sodium hydroxide solution to adjust the pH value of the above waste liquid to 11.5, and add 5 mg of polyacrylamide, preferably stir and react at a speed of 150 rpm for 2 minutes, then s...
Embodiment 3
[0063] Please refer to figure 1 Shown is the process flow diagram of the treatment method for analyzing waste liquid of the present invention. Potassium dichromate method measures the analytical waste liquid 1000ml that COD produces and is placed in 1# sedimentation tank, adds sodium chloride 2.0g to described waste liquid, leaves standstill after suitably stirred, Ag + Converted to silver chloride precipitation, filtration and separation, silver chloride recovery, and the waste liquid is transferred to 2# precipitation tank;
[0064] Use sodium hydroxide solution to adjust the pH value of the waste liquid in the 2# precipitation tank to 2.85, add 10 g of sodium metabisulfite and 30 g of sodium sulfite, preferably stir and react at a speed of 150 rpm for 30 min;
[0065] Use sodium hydroxide solution to adjust the pH value of the waste liquid to 11.8, and add 5 mg of polyacrylamide, preferably stir and react at a speed of 150 rpm for 2 minutes, then stir and react at a speed of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com