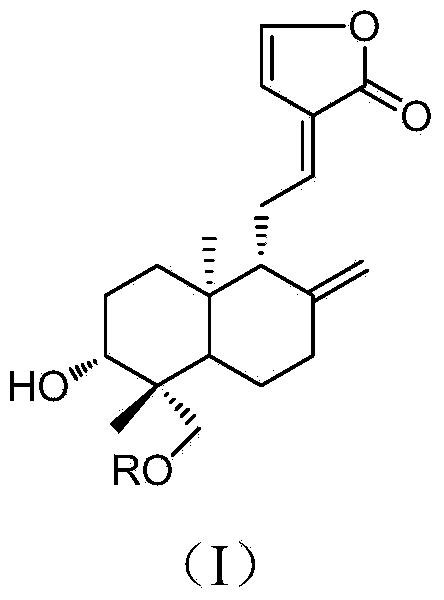
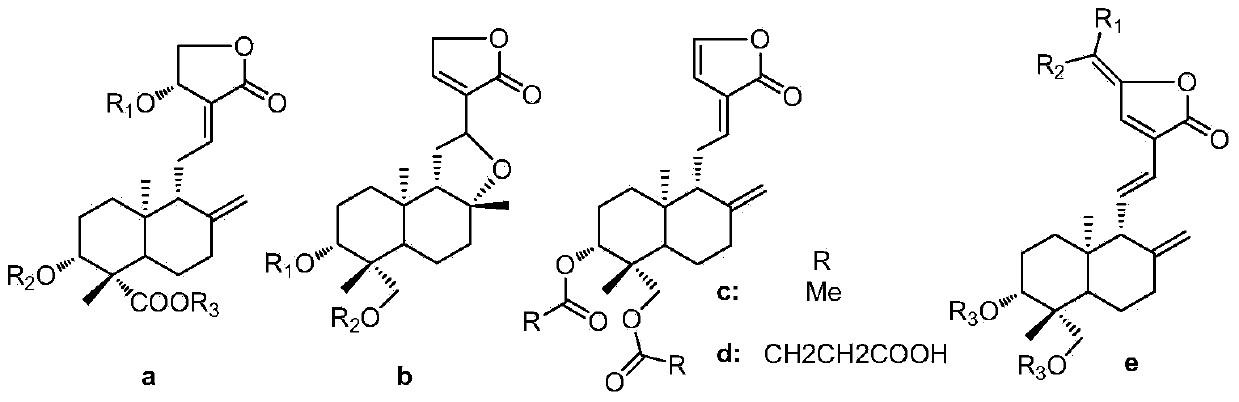
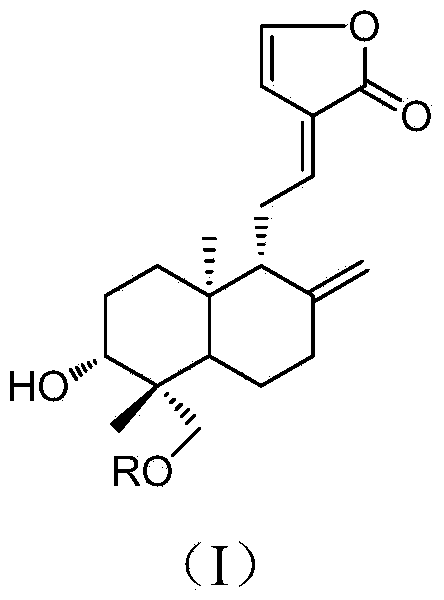
14-deoxy-14,15-didehydro andrographolide derivatives and medicine composition and application thereof
A technology of andrographolide, didehydrogenation, applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0022] Preparation of raw material andrographolide:
[0023] Take 10 kg of the whole plant of Andrographis paniculata and grind it, extract three times with 30 kg of 90% ethanol, each time for 3 hours, combine the ethanol extract, concentrate to a volume of 10 liters, extract the concentrated solution three times with ethyl acetate, each time 18 liters, combine acetic acid Ethyl extract, ethyl acetate was recovered to obtain 336 g of ethyl acetate extract.
[0024] 336 grams of ethyl acetate was dissolved in acetone, adsorbed on 600 grams of silica gel, evaporated to dryness at room temperature, passed through a 60-100 mesh sieve, and set aside. Take 3000 grams of column chromatography silica gel (200-300 mesh, Qingdao Meigao Company), and fill it in a glass chromatography column with a mixed solvent of ethyl acetate-petroleum ether (40:60) by wet method (column diameter 18cm, height of filled silica gel 28cm), after the sample was loaded, it was eluted with a mixed solvent o...
preparation Embodiment 2-3
[0036] According to Example 1, add 0.4mmol (200mg) andrographolide, 0.2mmol (34mg) 4-dimethylaminopyridine (DMAP) and 0.6mmol corresponding acid into a 10mL round bottom flask, then add 5ml of anhydrous N,N - Dimethylformamide, after stirring to dissolve, add 1.0mmol (206mg) N-dicyclohexylcarbodiimide (DCC) under ice-bath conditions and stir, and the reaction temperature rises to room temperature. After the reaction of andrographolide was detected by TLC, the reaction solution was filtered, washed three times with dilute hydrochloric acid, saturated aqueous sodium bicarbonate solution, and saturated saline solution, and then dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to obtain a crude product, which was passed through a silica gel column layer Analysis and eluting with ethyl acetate-petroleum ether (30:70-40:60) to prepare 14-deoxy-14,15-didehydroandrographolide derivatives (2-3) with the following structure:
[0037] Compound 2 and 3...
preparation Embodiment 1
[0075] According to the method of Preparation Example 1, 14-deoxy-14,15-didehydroandrographolide derivatives were first prepared, dissolved with a small amount of DMSO, added water for injection as usual, finely filtered, potted and sterilized to make injections .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com