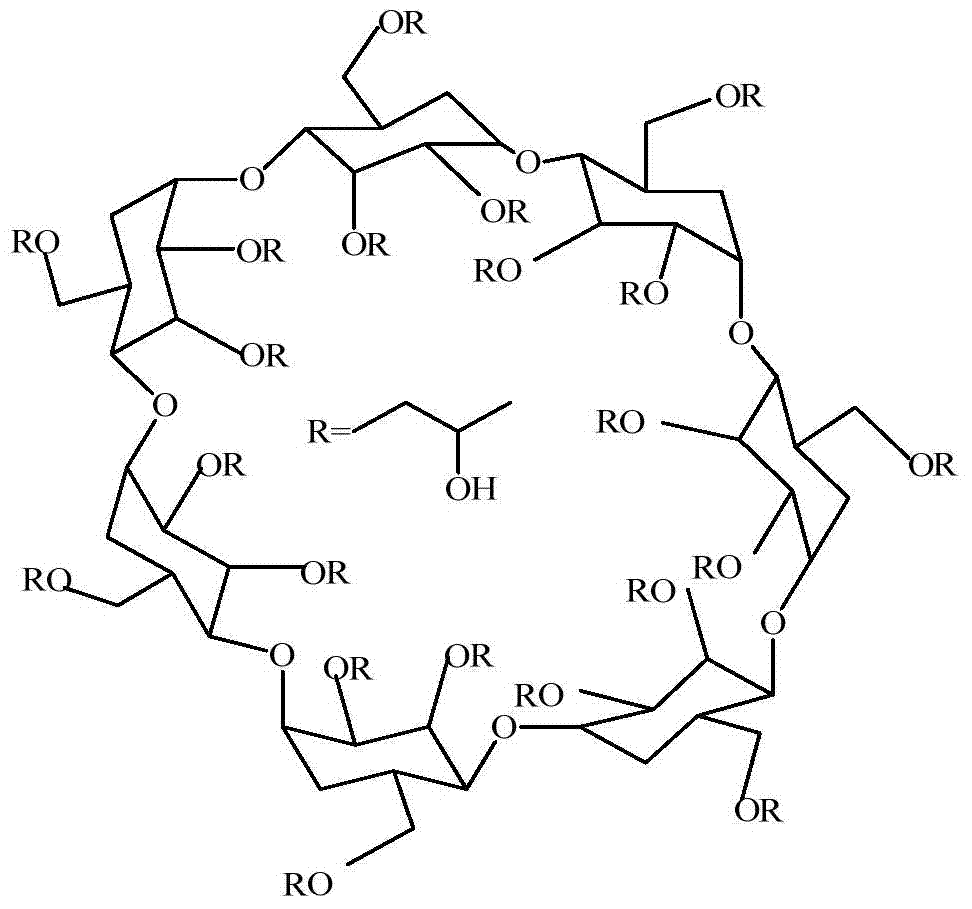
Hydroxypropyl-beta-cyclodextrin chiral composite membrane, and applications thereof
A chiral composite membrane and cyclodextrin technology, which is applied in membrane technology, semi-permeable membrane separation, climate sustainability, etc., can solve the problems that have not yet been reported in the public application of hydroxypropyl-β-cyclodextrin composite membrane , to achieve the effect of easy large-scale industrial production, low cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The commercial polysulfone membrane is used as the base membrane, soaked in deionized water for two days, dried vertically, soaked in 0.02g / mL hydroxypropyl-β-cyclodextrin solution for 3h, taken out and dried vertically. Interfacial polymerization with 0.012g / mL 1,6-hexamethylene diisocyanate heptane solution, polymerization time 10s. Take out the polysulfone membrane, wait for the surface reagent to volatilize, put it in a drying oven at 110°C, and heat-treat it for 20 minutes. The polysulfone composite membrane is washed with deionized water to obtain the required hydroxypropyl-β-cyclodextrin chiral composite membrane.
Embodiment 2
[0035] The commercial polysulfone membrane is used as the base membrane, soaked in deionized water for two days, dried vertically, soaked in 0.02g / mL hydroxypropyl-β-cyclodextrin solution for 3h, and taken out vertically for 0.5h. Perform interfacial polymerization with 0.021g / mL1,3,5-tribenzoylchloroheptane solution, and the polymerization time is 20s. Take out the polysulfone membrane, wait for the surface reagent to volatilize, put it in a drying oven at 110°C, and heat-treat it for 25 minutes. The polysulfone composite membrane is washed with deionized water to obtain the required hydroxypropyl-β-cyclodextrin chiral composite membrane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com