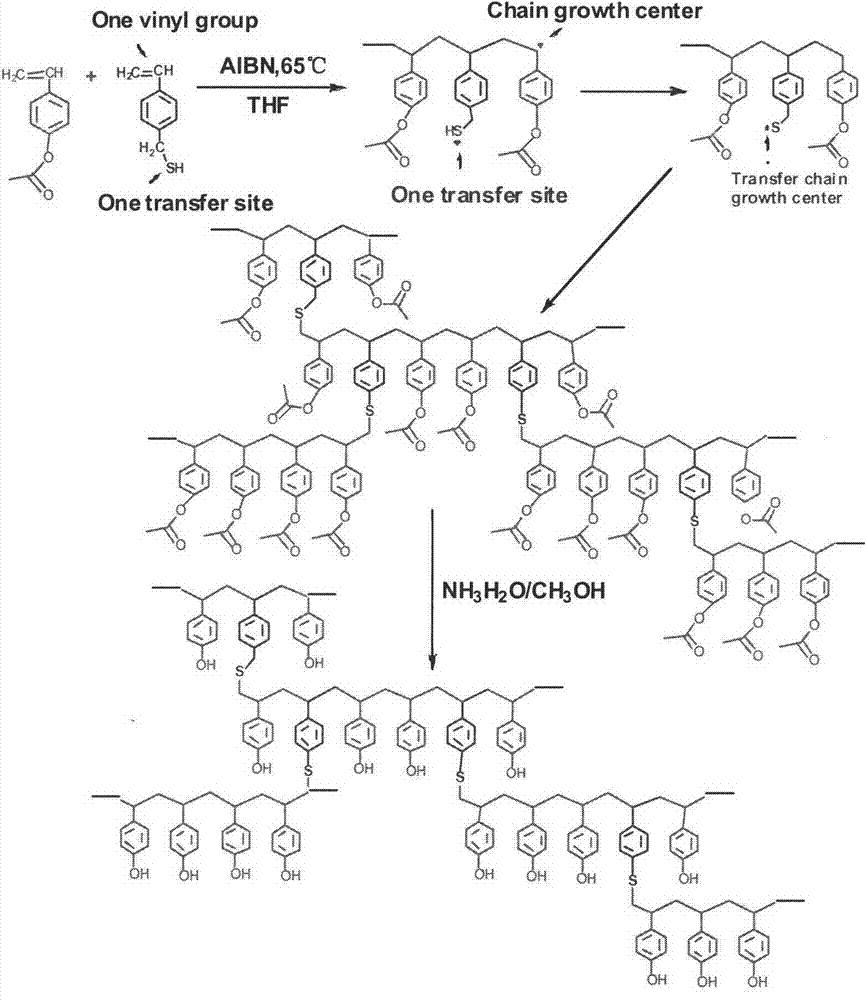
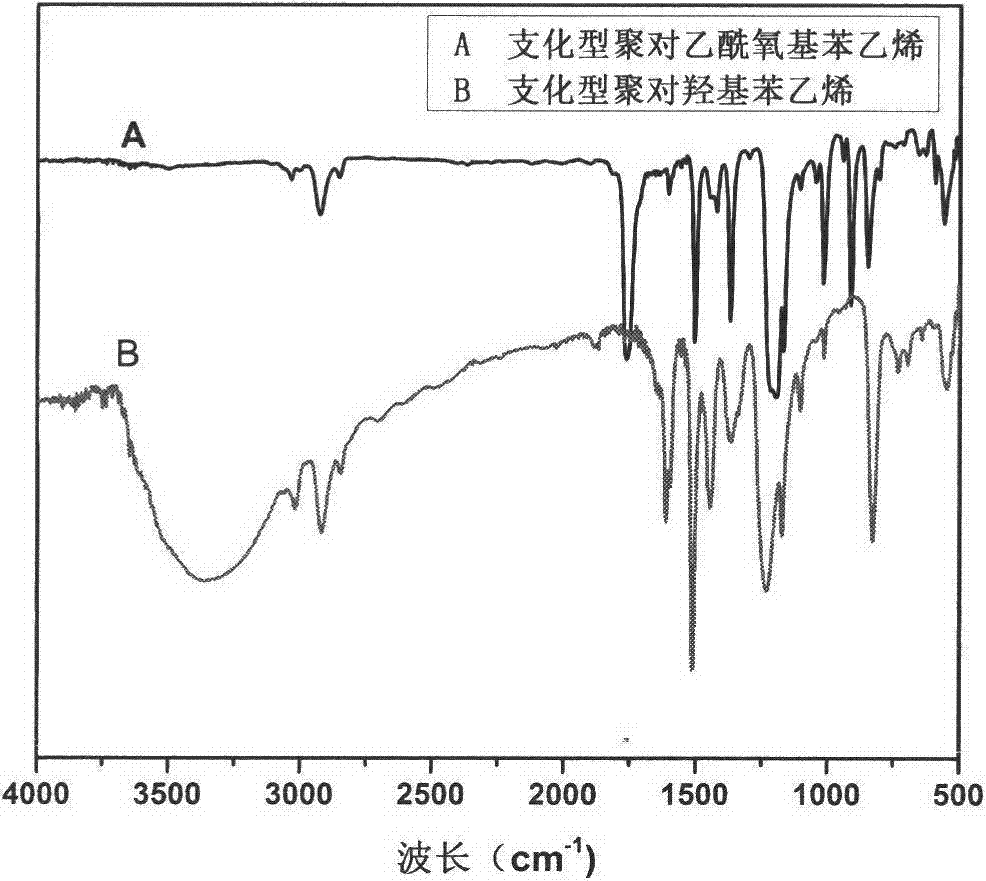
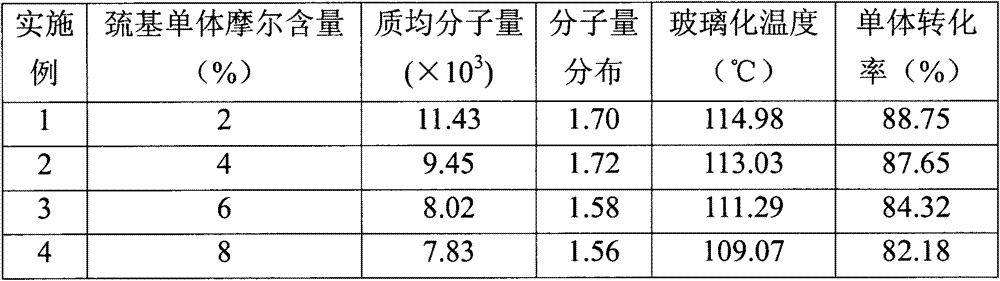
Preparation method of branched poly (p-hydroxystyrene)
A technology of hydroxystyrene and acetoxystyrene is applied in the field of preparation of branched poly(p-hydroxystyrene) to achieve the effects of simple preparation operation, controllable structure and performance, and mild polymerization conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of branched poly(p-acetoxystyrene): add (22mmol) 3.58g p-acetoxystyrene, ( (0.44mmol) 0.08g p-vinylbenzylmercaptan, (0.44mol) 0.072g AIBN, and then add 40ml of tetrahydrofuran as a polymerization solvent, place the flask in a constant temperature oil bath, heat and stir to 40°C, and then seal it with nitrogen gas for 10 minutes system, the temperature was raised to 65°C, the reaction was stirred at constant temperature for 24 hours, and then cooled to room temperature. Add the obtained polymer solution dropwise to methanol for sedimentation and suction filtration. After suction filtration, the obtained solid is repeatedly dissolved, precipitated and purified, and the obtained solid is vacuum-dried to obtain a branched poly(p-acetoxystyrene) product.
[0026] (2) Deacetoxylation of branched poly(p-acetoxystyrene): add 1.5g branched poly(p-acetoxystyrene) and 35ml methanol to a 100ml three-necked flask, 7.45g of ammonia water (containing 3g of ammonium hydr...
Embodiment 2
[0028] (1) Synthesis of branched poly(p-acetoxystyrene): add (22mmol) 3.58g p-acetoxystyrene, ( (0.88mmol) 0.16g p-vinylbenzylmercaptan, (0.44mol) 0.072g AIBN, and then add 40ml of tetrahydrofuran as a polymerization solvent, place the flask in a constant temperature oil bath, heat and stir to 40°C, and then seal it after nitrogen gas for 10min system, the temperature was raised to 65°C, the reaction was stirred at constant temperature for 24 hours, and then cooled to room temperature. Add the obtained polymer solution dropwise to methanol for sedimentation and suction filtration. After suction filtration, the obtained solid is repeatedly dissolved, precipitated and purified, and the obtained solid is vacuum-dried to obtain a branched poly(p-acetoxystyrene) product.
[0029] (2) Deacetoxylation of branched poly(p-acetoxystyrene): add 1.5g branched poly(p-acetoxystyrene) and 35ml methanol to a 100ml three-necked flask, 7.45g of ammonia water (containing 3g of ammonium hydroxid...
Embodiment 3
[0031] (1) Synthesis of branched poly(p-acetoxystyrene): add (22mmol) 3.58g p-acetoxystyrene, ( (1.32mmol) 0.24g p-vinylbenzylmercaptan, (0.44mol) 0.072g AIBN, and then add 40ml tetrahydrofuran as a polymerization solvent, place the flask in a constant temperature oil bath, heat and stir to 40°C, and then seal it with nitrogen gas for 10 minutes system, the temperature was raised to 65°C, the reaction was stirred at constant temperature for 24 hours, and then cooled to room temperature. Add the obtained polymer solution dropwise to methanol for sedimentation and suction filtration. After suction filtration, the obtained solid is repeatedly dissolved, precipitated and purified, and the obtained solid is vacuum-dried to obtain a branched poly(p-acetoxystyrene) product.
[0032] (2) Deacetoxylation of branched poly(p-acetoxystyrene): add 1.5g branched poly(p-acetoxystyrene) and 35ml methanol to a 100ml three-necked flask, 7.45g of ammonia water (containing 3g of ammonium hydroxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com