Controllable preparation method of carbazole bromo-compound
A compound, the technology of carbazole bromide, which is applied in the field of controllable preparation of carbazole bromide compounds, achieves the effects of high yield, mild reaction conditions and easy realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
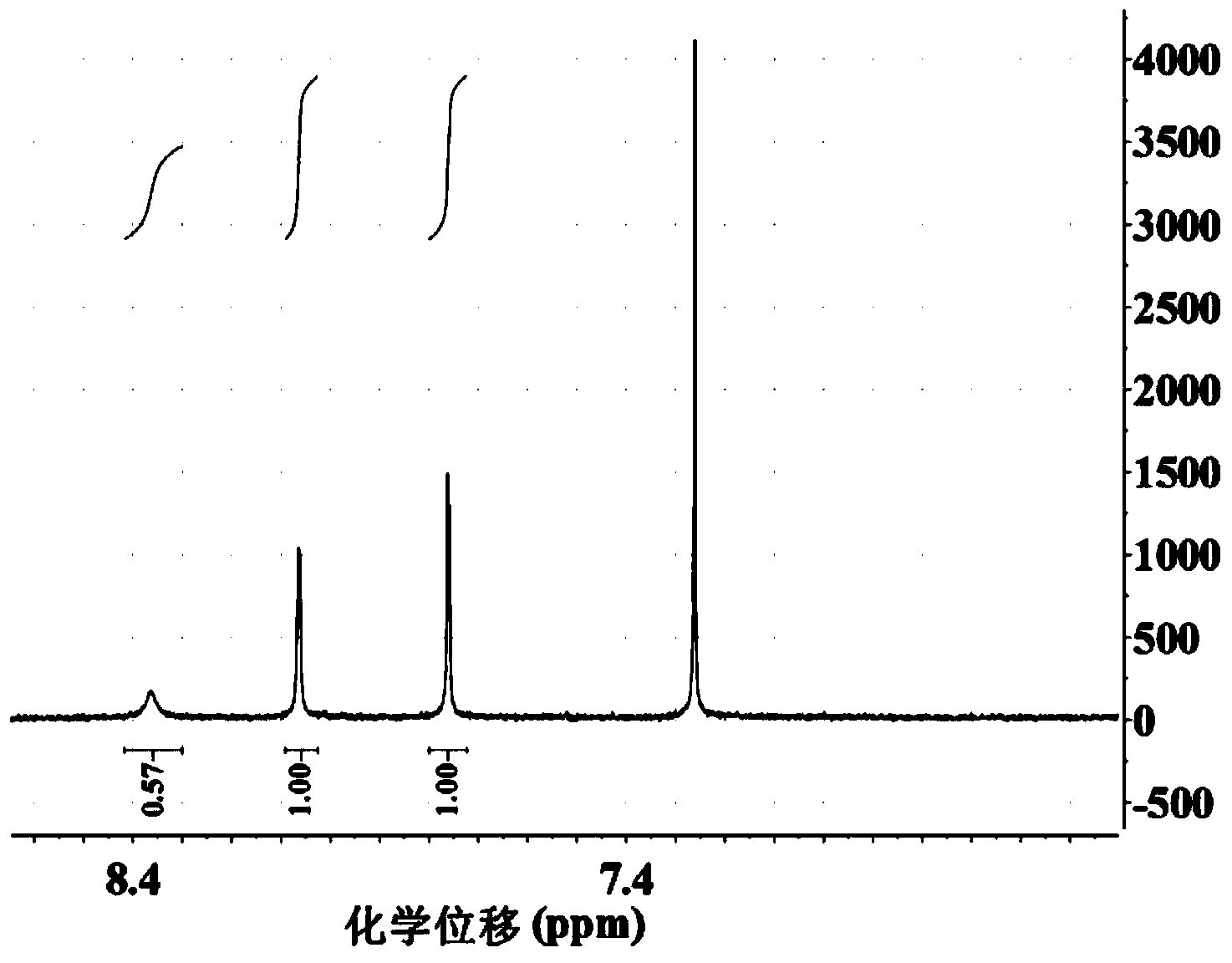
Embodiment 1
[0030] Using carbazole as the starting material, react with N-bromosuccinimide to synthesize 1,3,6,8-tetrabromocarbazole compound:
[0031]
[0032] Reaction Scheme
[0033]
[0034] Step 1: Under a nitrogen atmosphere, add 5 g (29.94 mmol) of carbazole and 15 mL of N,N-diformamide to a three-necked flask equipped with magnetic stirring. After the carbazole is completely dissolved, the prepared concentration is 1.996 mol / For solution A of L, reduce the reaction system to 5°C with an ice-water bath; N-bromosuccinimide (21.317 g, 119.76 mmol) was dissolved in 100 mL N,N-diformamide to prepare a concentration of 1.1976 mol / L solution B, the ratio of the amount of N,N-diformamide in solution B to the amount of N,N-diformamide in solution A is 20:3;
[0035] Step 2: Control the reaction temperature at 5°C, slowly add solution B to solution A slowly with a constant pressure funnel;
[0036] Step 3: After the dropwise addition, react at room temperature for 10 hours;
[00...
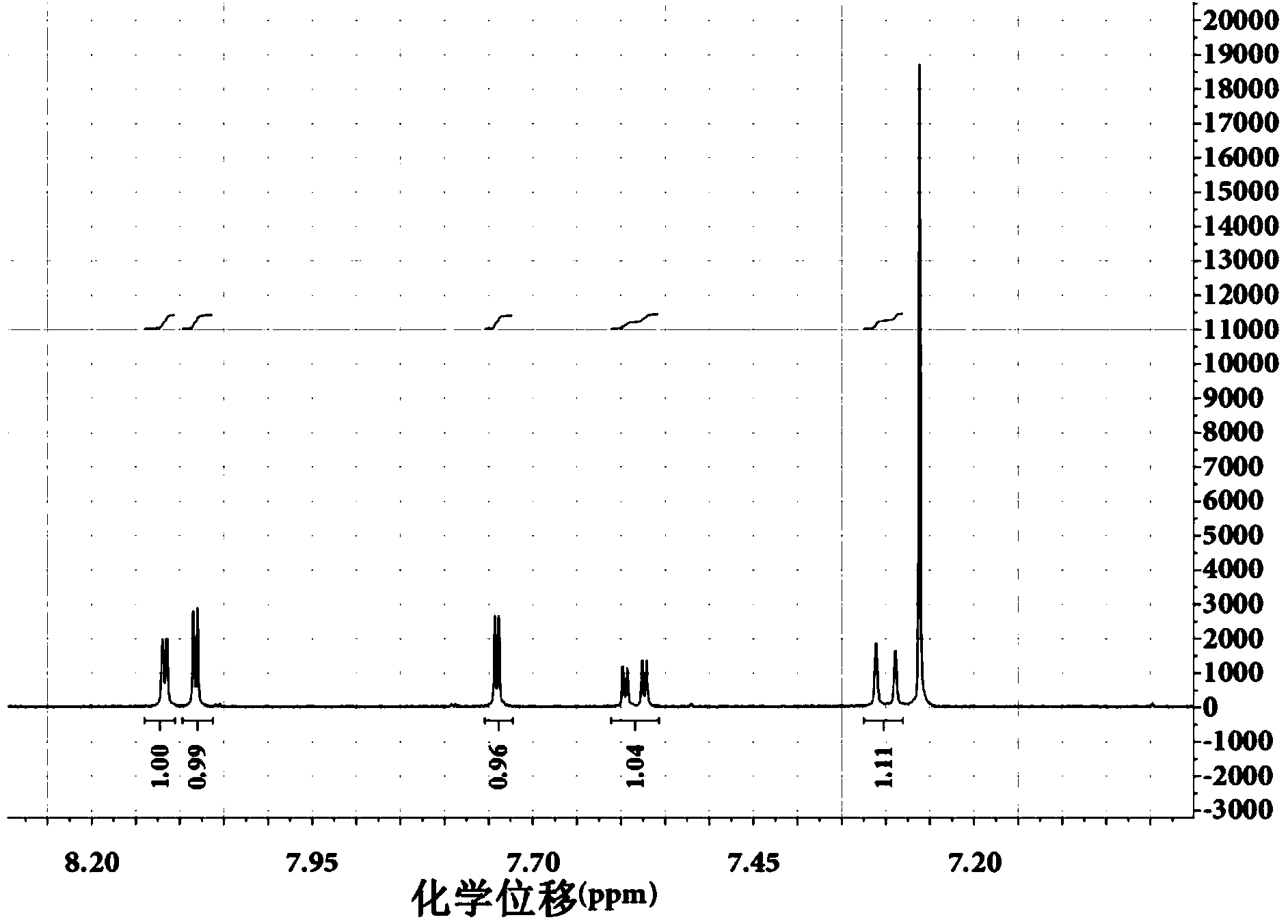
Embodiment 2
[0040] Using carbazole as the starting material, react with N-bromosuccinimide to synthesize 1,3,6-tribromocarbazole:
[0041]
[0042] Reaction Scheme
[0043]
[0044] Step 1: Under a nitrogen atmosphere, add 5 g (29.94 mmol) of carbazole and 15 mL of N,N-diformamide to a three-necked flask equipped with magnetic stirring. After the carbazole is completely dissolved, the prepared concentration is 1.996 mol / For solution A of L, the reaction system was lowered to 3°C with an ice-water bath; N-bromosuccinimide (15.898 g, 89.82 mmol) was dissolved in 100 mL N,N-diformamide to prepare a concentration of 0.8932 mol / L solution B, the ratio of the amount of N,N-diformamide in solution B to the amount of N,N-diformamide in solution A is 20:3;
[0045] Step 2: Control the reaction temperature at 3°C, and slowly add solution B to solution A dropwise with a constant pressure funnel;
[0046] Step 3: After the dropwise addition, react at room temperature for 10 hours;
[0047] S...
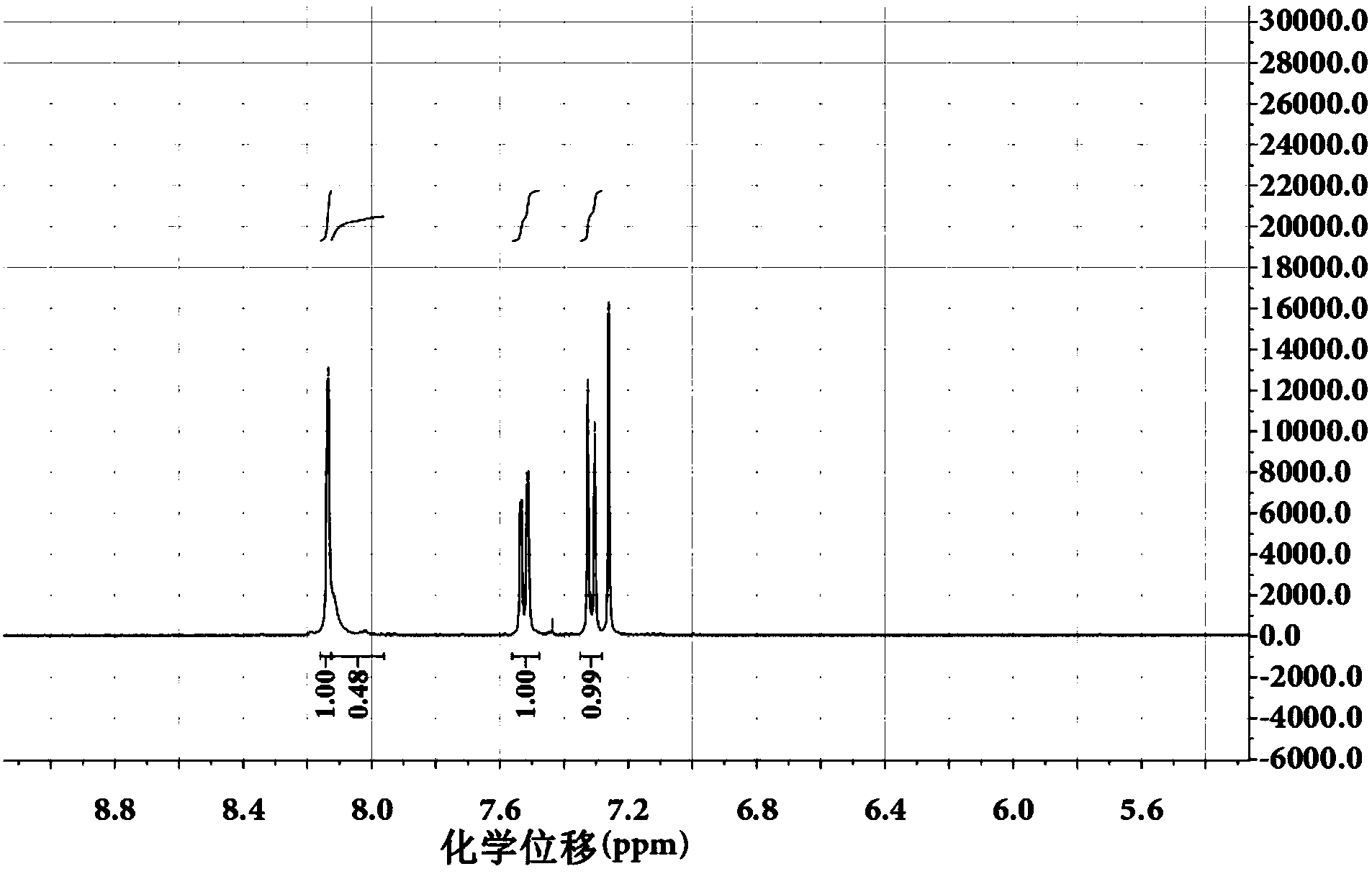
Embodiment 3
[0051] Using carbazole as the starting material, react with N-bromosuccinimide to synthesize 3,6-dibromocarbazole:
[0052]
[0053] Scheme of the reaction scheme:
[0054]
[0055] Step 1: Under a nitrogen atmosphere, add 5 g (29.94 mmol) of carbazole and 15 mL of N,N-diformamide to a three-necked flask equipped with magnetic stirring. After the carbazole is completely dissolved, the prepared concentration is 1.996 mol / For solution A of L, reduce the reaction system to 0°C with an ice-water bath; N-bromosuccinimide (10.601 g, 59.84 mmol) was dissolved in 60 mL N,N-diformamide to prepare a concentration of 0.9973 mol / L solution B, the ratio of the amount of N,N-diformamide in solution B to the amount of N,N-diformamide in solution A is 4:1;
[0056] Step 2: Control the reaction temperature at 0°C, and slowly add solution B to solution A slowly with a constant pressure funnel;
[0057] Step 3: After the dropwise addition, react at room temperature for 10 hours;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com