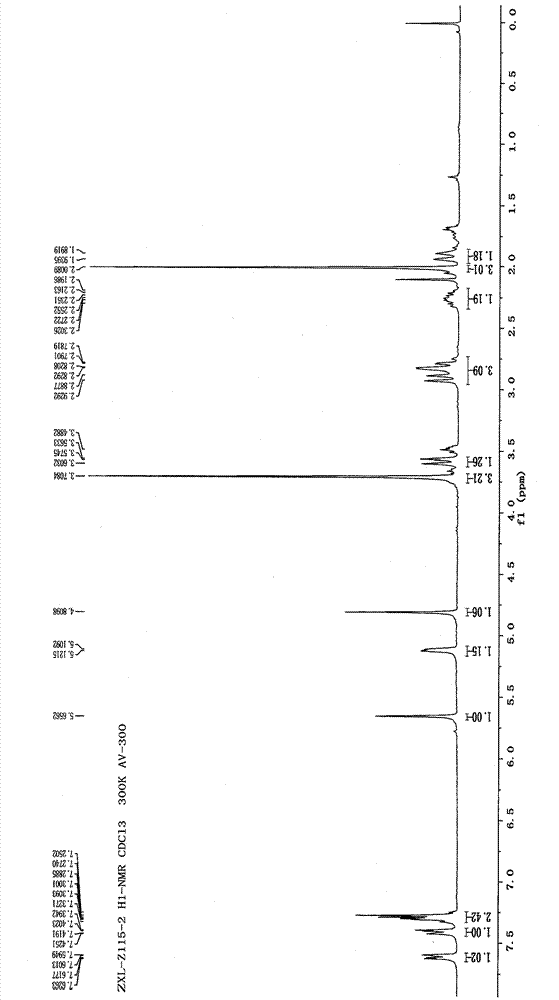
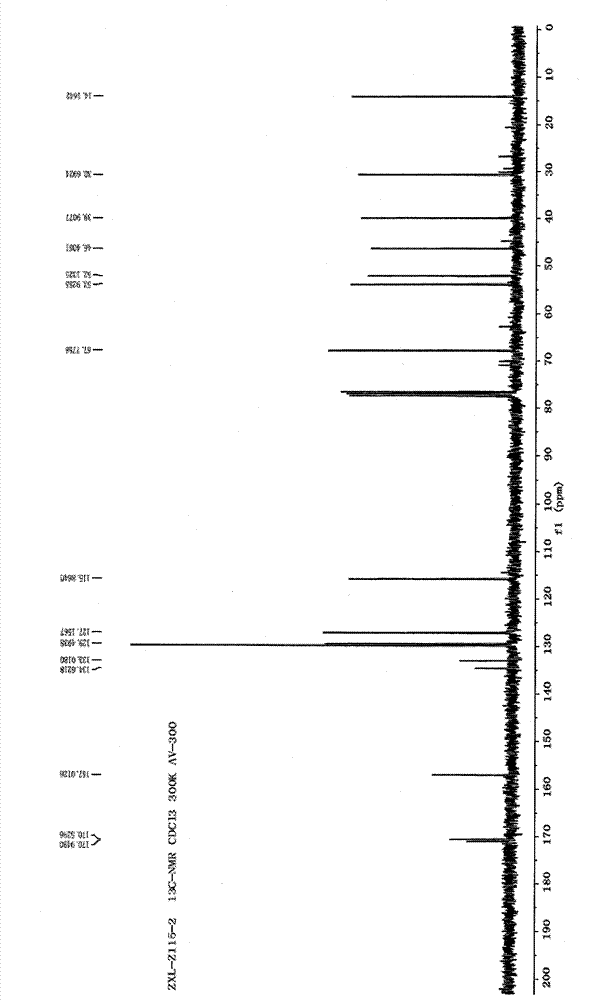
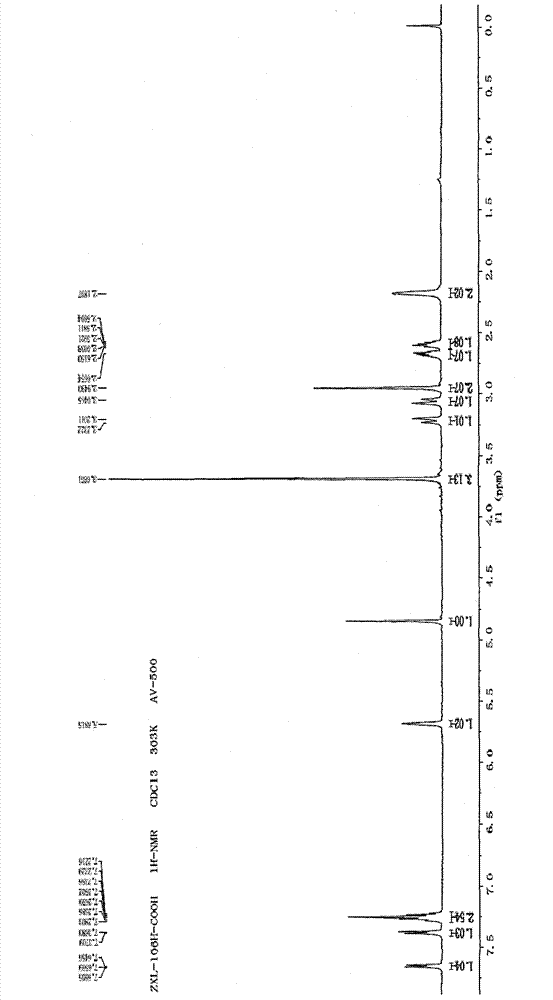
Metabolin marker of 2-hydroxyl radical tetrahydro-thiophene pyridine derivative with optical activity as well as preparation and application thereof
A technology for metabolites and uses, applied in the field of preparation of novel metabolite markers, can solve problems such as drug efficacy and safety uncertainty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Pharmacokinetic study of vicagrel in cynomolgus monkeys, beagle dogs and SD rats
[0049] 1. Purpose of the test: To identify the metabolites in the plasma of cynomolgus monkeys, beagle dogs and SD rats after intragastric administration of vicagrelor, and to compare the differences in drug metabolism in vivo.
[0050] 2. Sample collection: 2 healthy cynomolgus monkeys, 1 male and 1 male, each weighing 3-4 kg, were given vicagrelor by intragastric administration at 5 mg / kg. Two healthy Beagle dogs, male, weighing 8-10 kg, were given Vicarrel by gavage at 5 mg / kg. Two healthy SD rats, male, weighing 180-220 g, were given vicagrelor by intragastric administration at 10 mg / kg. Fasting for 12 hours before the test, drinking water freely, taking medicine on an empty stomach in the morning, and eating uniformly 2 hours after taking the medicine. Take 0.5ml of venous blood before administration and 0.5, 1, 4 and 8h after administration, and add 25μl of derivatization reagent ...
Embodiment 2
[0063] Research on the metabolic behavior of vicagrelor in human body
[0064] 1. Purpose of the test: To use UPLC / Q-TOF MS to qualitatively and quantitatively identify the metabolites in the plasma of voluntary subjects after oral administration of vicagrel tablets.
[0065] 2. Materials and methods
[0066] Reagent:
[0067] 3'-Methoxybromoacetophenone
American Sigma Corporation
Acetonitrile (chromatographically pure)
American Sigma Corporation
Ammonium acetate (chromatographically pure)
American Tedia Corporation
American Fluka Corporation
[0068] Sample collection: 3 healthy male voluntary subjects, fasted for 12 hours before the test, took 25 mg of vicagrelor with 200ml of warm water on the day of the test, and could drink water 2 hours after taking the medicine, and eat after 4 hours. Before taking the medicine (0h) and 1, 2 and 6h after taking the medicine, 2 mL of blood was collected. Immediately after...
Embodiment 3
[0081] Pharmacokinetic study of compound of formula I-3 in SD rats
[0082] 1. Purpose of the test: To identify the metabolites in the plasma of SD rats given the compound of formula I-3 by intragastric administration, and to study the drug metabolism behavior in vivo.
[0083] 2. Sample collection: 2 healthy SD rats, male, weighing 180-220 g, were intragastrically administered the compound of formula I-3 at 10 mg / kg. Fasting for 12 hours before the test, drinking water freely, taking medicine on an empty stomach in the morning, and eating uniformly 2 hours after taking the medicine. Take 0.5 ml of venous blood before administration and 1, 4 and 8 hours after administration, and immediately add 25 μl of derivatization reagent (3'-methoxybromoacetophenone (BMP) acetonitrile solution) in EDTA In the anticoagulant tube, gently invert the test tube 5-6 times as soon as possible, place at room temperature for 10 minutes, centrifuge at 3500rpm for 10 minutes, separate the plasma, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com