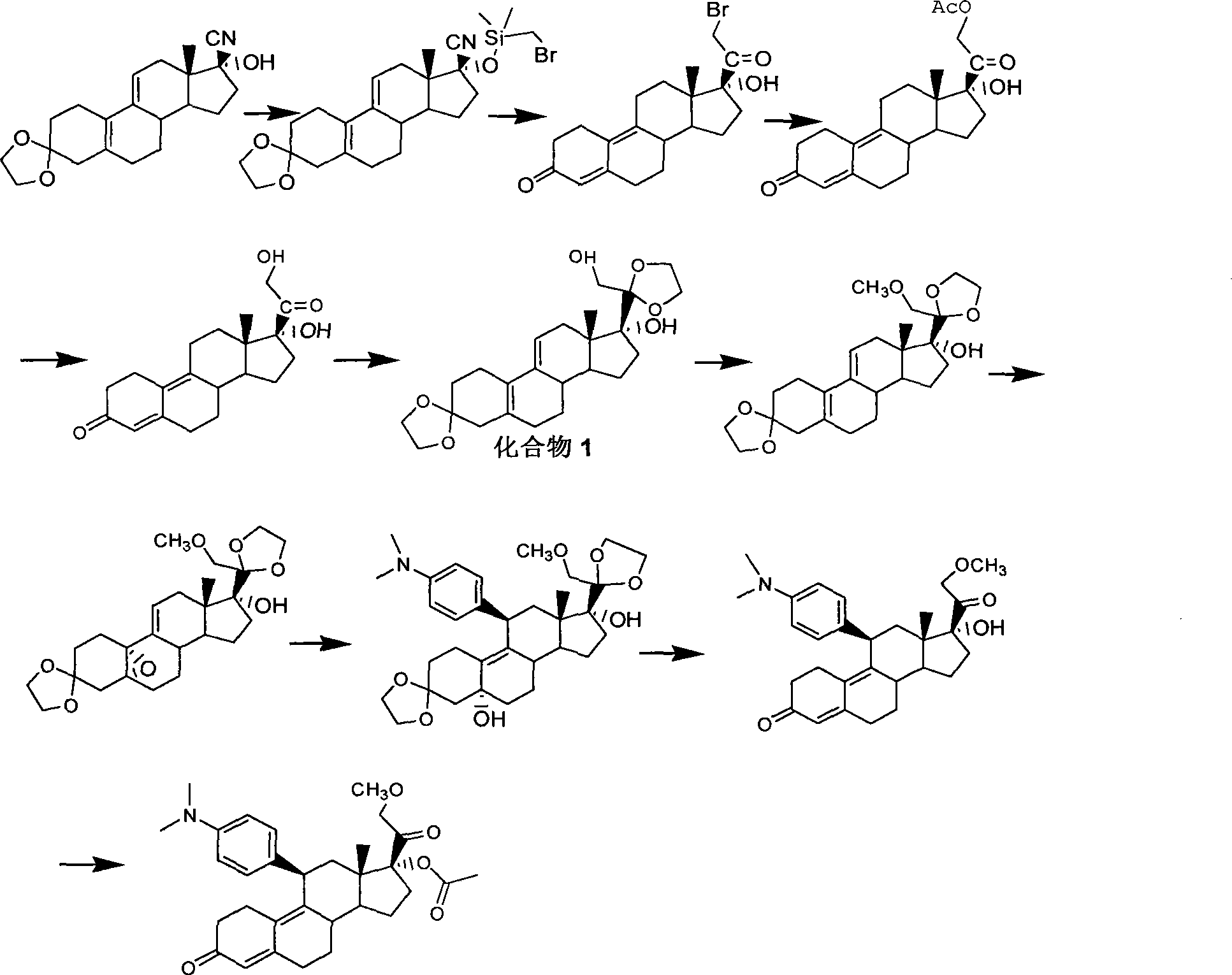
Preparation methods and purposes of Proellex(R)-V and intermediate of Proellex(R)-V
The technology of a diketone and steroid compound is applied in the field of preparation and use of trapristone acetate and its intermediates, which can solve problems such as being unfavorable to industrialized production, and achieve the advantages of being beneficial to industrialized production, easy to obtain raw materials and reagents, and simple raw materials and reagents. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
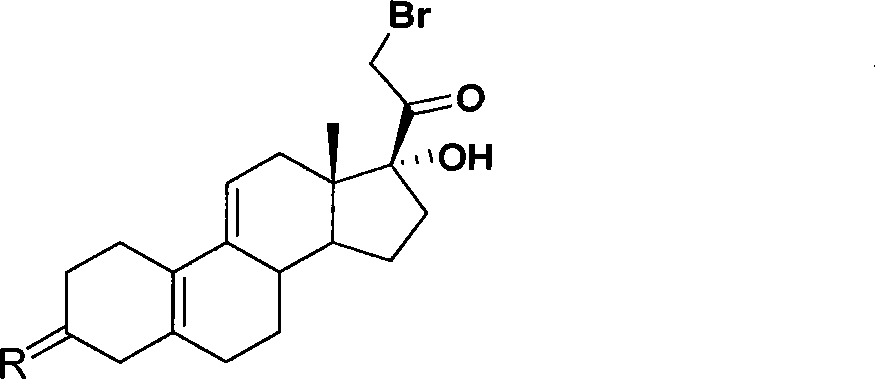
[0034] Example 1: 17α-Hydroxy-21-bromo-19-norpregna-5(10),9(11)-diene-3,20-dione
[0035] In a dry reaction flask, add 20g of compound 17α-hydroxy-20-methoxy-19-norpregna-5(10), 9(11), 20-triene-3-one, and then add 200mL Dichloromethane and 5mL triethylamine were stirred to dissolve, and the temperature was lowered to about 0°C. An appropriate amount of about 10% bromodichloromethane solution was added dropwise into the reaction flask, and the reaction was kept warm, and the reaction of the raw materials was monitored by TLC until complete. Slowly pour the reaction system into 100 mL of cooled 1 mol / L hydrochloric acid solution, let stand, separate the two phases, extract the aqueous phase with an appropriate amount of dichloromethane, combine the organic phases, and wash once with saturated brine. Dry over anhydrous magnesium sulfate, filter, and rinse the filter cake with an appropriate amount of dichloromethane. The combined filtrates were concentrated under reduced press...
Embodiment 2
[0036] Example 2: 3,3-Dimethoxy-17α-hydroxy-21-bromo-19-norpregna-5(10),9(11)-dien-20-one
[0037] In a dry reaction flask, add 20 g of compound 3,3-dimethoxy-17α-hydroxy-20-methoxy-19-norpregna-5(10), 9(11), 20-triene , and then add 200mL of dichloromethane and 50mL of triethylamine in sequence, stir to dissolve, and cool down to about 0°C. An appropriate amount of about 10% bromodichloromethane solution was added dropwise into the reaction flask, and the reaction was kept warm, and the reaction of the raw materials was monitored by TLC until complete. Slowly pour the reaction system into 100 mL of cooled saturated sodium chloride solution, let it stand, separate the two phases, extract the aqueous phase with an appropriate amount of dichloromethane, combine the organic phases, and wash once with saturated brine. Dry over anhydrous magnesium sulfate, filter, and rinse the filter cake with an appropriate amount of dichloromethane. The combined filtrates were concentrated und...
Embodiment 3
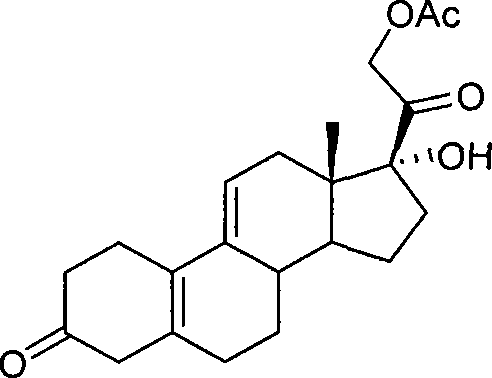
[0038] Example 3: 17α-Hydroxy-21-acetoxy-19-norpregna-5(10),9(11)-diene-3,20-dione
[0039] Add the compound 2 prepared in Example 1 into the dry reaction bottle, add 400 mL of acetone, protect it under nitrogen, stir at room temperature, then add 50 g of potassium acetate, 34 g of potassium iodide, and 1.2 mL of acetic acid in sequence. The temperature was raised to reflux, and the raw materials were monitored by TLC to complete the reaction. Cool down to room temperature, filter, rinse the filter cake once with a small amount of acetone, combine the filtrates, and concentrate to dryness under reduced pressure. Add dichloromethane to dissolve, then wash with saturated brine and water successively, dry over anhydrous magnesium sulfate, filter, rinse the filter cake once with a small amount of dichloromethane, combine the filtrates, and concentrate to dryness under reduced pressure to obtain about 19.2 g of the target product 17α-Hydroxy-21-acetoxy-19-norpregna-5(10),9(11)-die...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com