Spiral polyallylene containing azobenzene dipoles and preparation method thereof
A technology of azobenzene and polypropyne, which is applied in the field of helical polypropyne and its preparation, can solve the problems of limiting the application range of helical polyacetylene and unstable helical structure, and achieves the effect of stabilizing the helical structure and facilitating the helical structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] The synthesis of poly(N-ethyl-N-ethylpentanoate) phenylazobenzamide-L-alanine propynylamide is as follows:
[0040] a. In a 100 mL three-necked flask, add 3.3 g (20 mmol) N-ethyl-N-hydroxyethylaniline, 2.75 mL (30 mmol) valeric acid, and 30 mL treated anhydrous DMF in sequence. Under nitrogen protection, stir under ice bath to dissolve, then add 0.366g (3mmol) DMAP, 6.18g (30mmol) DCC, and stir overnight at room temperature. The solvent was distilled off under reduced pressure, a small amount of water was added to the residue to wash it several times, and the white insoluble solid was filtered off after dissolving with dichloromethane, and the filtrate was concentrated under reduced pressure and separated by silica gel column chromatography (methanol / dichloromethane=1 : 30 (V / V)), the component with the highest concentration was collected, and the solvent was distilled off under reduced pressure to obtain 4.01 g of light yellow product N-ethyl-N-pentanoic ac...
Embodiment 2
[0047]
[0048] Synthesis of poly(N-ethyl-N-ethyl pentanoate) phenylazobenzamide-L-alanine propynyl ester: the preparation conditions in steps a-c are the same as in Example 1, steps d, e as follows:
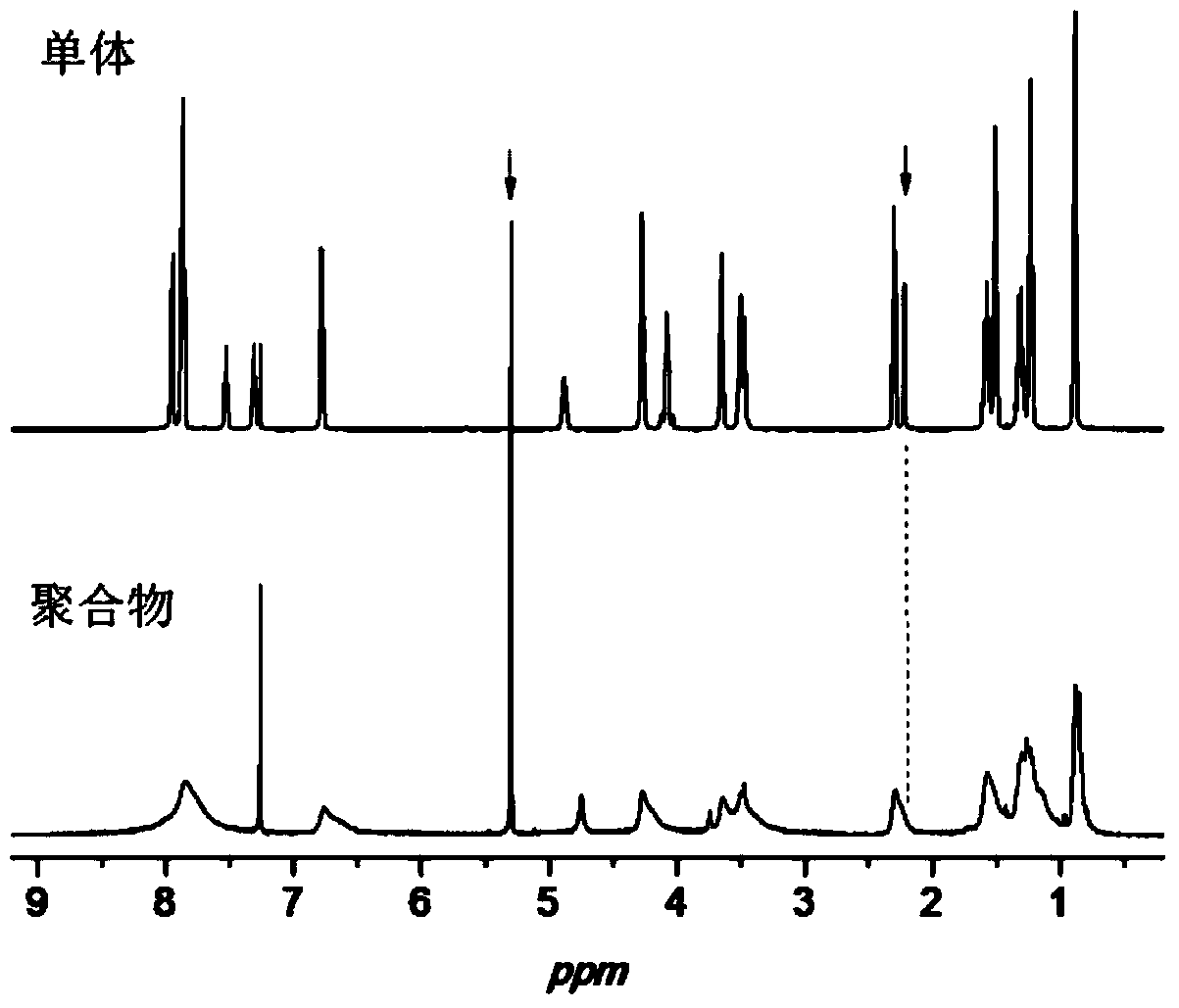
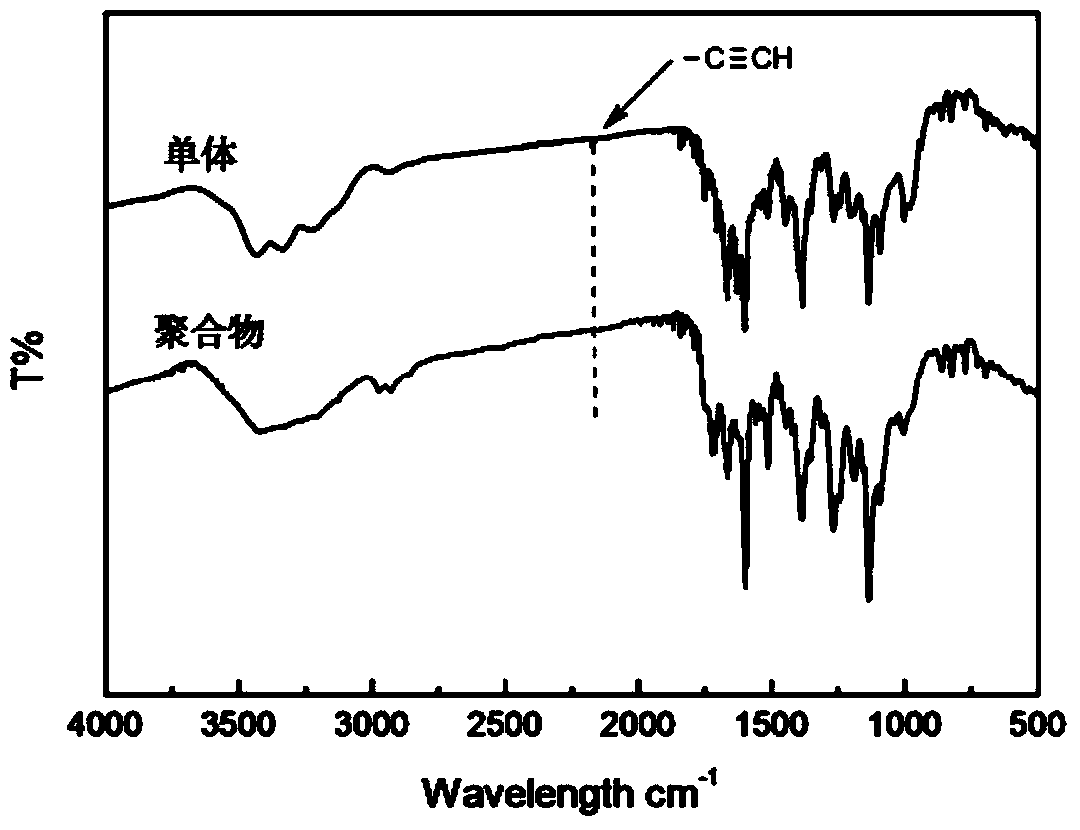
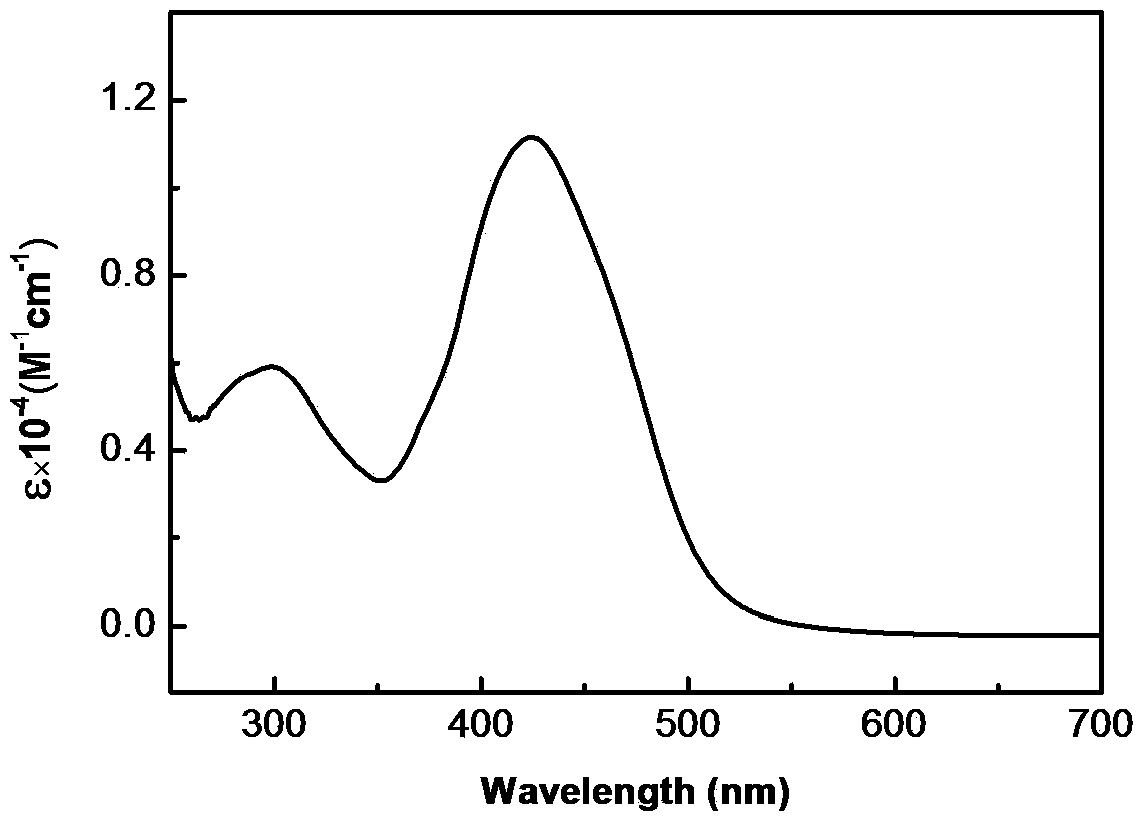
[0049] d, the difference from Example 1 is that the propyne compound is propynyl alcohol, the quality of N-ethyl-N-pentanoic acid ethyl) phenyl azobenzamide-L-alanine and DMF The ratio is 1:9, and the molar ratio of (N-ethyl-N-ethylpentanoate) phenylazobenzamide-L-alanine to propynyl alcohol and DCC is 1:1.2:1.8. Other conditions are the same as in Example 1. 1 HNMR (CDCl 3 , 400MHz) δ (ppm): 0.89 (t, 3H, CH 3 ), 1.23(t, 3H, CH 3 ), 1.29-1.36 (m, 2H, CH 2 ), 1.52(t, 3H, CH3), 1.56-1.61(m, 2H, CH2), 2.27(s, 1H, CH), 2.32(t, 2H, CH 2 ), 3.51(q, 2H, CH 2 ), 3.67(t, 2H, CH 2 ), 3.88 (s, 2H, CH 2 ), 4.25(t, 2H, CH 2), 4.88-4.91 (m, 1H, CH), 6.79-6.81 (m, 3H, NH, Ar-H), 7.85-7.97 (m, 6H, Ar-H). IR (KBr, cm -1 ): 3300~3495(N-H), 3327(≡C-H), 2169(C≡C), 1656(C=O), 1448(N=N...
Embodiment 3
[0052]
[0053] Synthesis of poly(N-ethyl-N-(N-acetylglycinate ethyl))phenylazobenzamide-L-alanine propynyl ester:
[0054] A, the difference with embodiment 1 is that raw material carboxylic acid is N-acetylglycine, condensing agent, additive are EDCI and HOBT, and the mass ratio of N-ethyl-N-hydroxyethylaniline and anhydrous DMF is: 1:19, the molar ratio of EDCI to HOBT is: 10:1. Other conditions are the same as in Example 1.
[0055] b. The difference from Example 1 is that the raw material N-ethyl-N-carboxylate ethyl aniline is (N-ethyl-N-(N-acetylglycine ethyl)) aniline, N-ethyl The molar ratio of base-N-(N-acetylglycine ethyl)aniline to p-aminobenzoic acid is: 1:1.2, after the diazonium salt of benzoic acid is added dropwise, the reaction is continued for 2h to obtain (N-ethyl-N- (N-acetylglycine ethyl carboxyl))phenylazobenzoic acid. Other conditions are the same as in Example 1.
[0056] c. The difference from Example 1 is that the raw material azobenzoic acid i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com