Atenolol pH independent sustained-release tablets and preparation method thereof
An atenolol-independent technology, applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as complex process and adverse recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
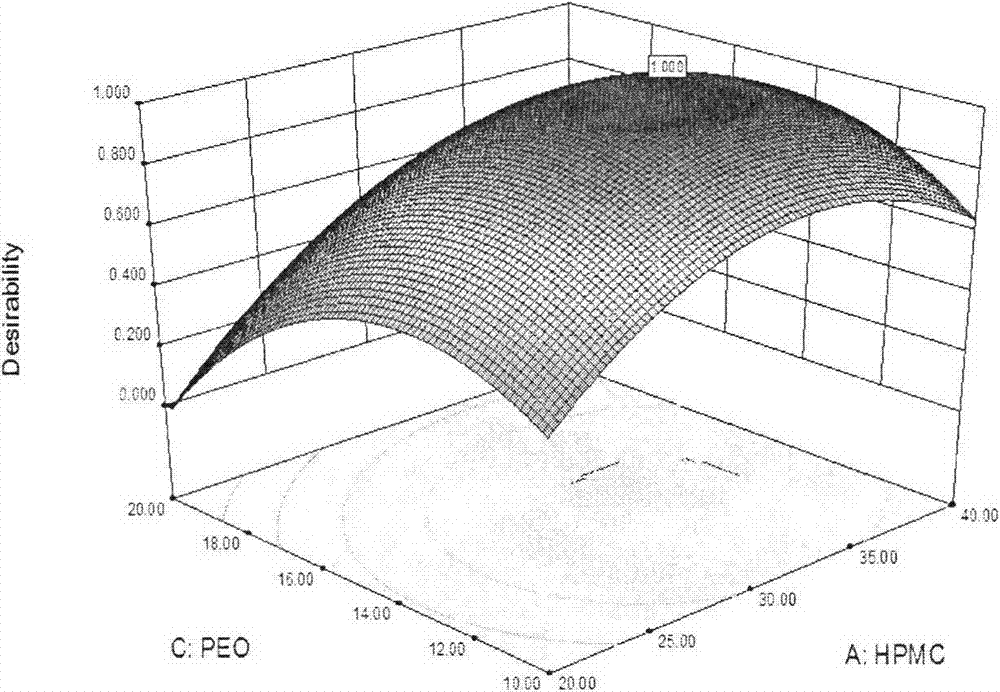
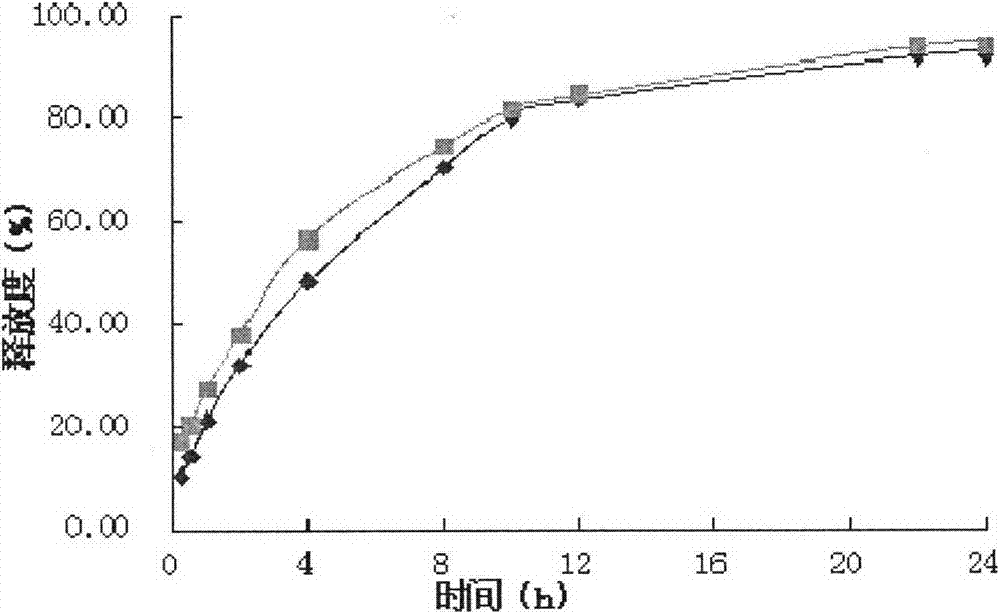
Image
Examples
Embodiment 1
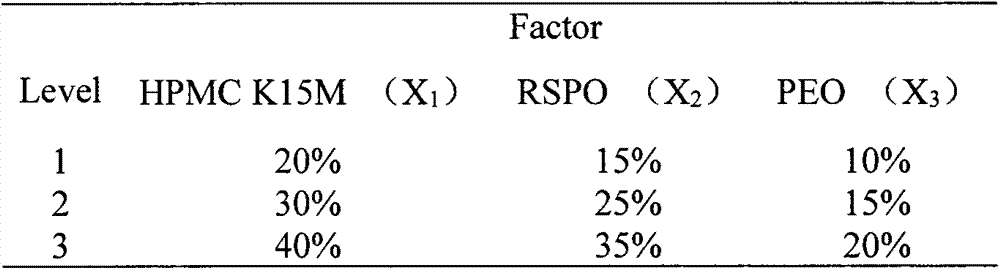
[0043] Atenolol pH-independent sustained-release tablet unit prescription (1000 tablets, 25mg per tablet). It contains the following components and weight:
[0044] Atenolol 25g
[0045] HPMC K15M 92.5g
[0046] Utraki RSPO 55g
[0047] PEO(POLYOX) 32.5g
[0048] Lactose 42.5g
[0049] Micronized silica gel and magnesium stearate 2.5g
[0050] The preparation method of the above-mentioned atenolol pH-independent sustained-release tablets is to mix the prescription amount of atenolol, HPMCK15M, Eudragit RSPO, high viscosity PEO, and lactose monohydrate in equal amounts. Then add 1% magnesium stearate and micro-powdered silica gel as lubricants, and adopt the powder direct pressing process to obtain atenolol pH-independent sustained-release tablets.
Embodiment 2
[0052] Atenolol pH-independent sustained-release tablet unit prescription (1000 tablets, 25mg per tablet). It contains the following components and weight:
[0053] Atenolol 25g
[0054] HPMC K15M 52.5g
[0055] Utraki RSPO 87.5g
[0056] PEO(POLYOX) 40g
[0057] Lactose 42.5g
[0058] Micronized silica gel and magnesium stearate 2.5g
[0059] The preparation method of the above-mentioned atenolol pH-independent sustained-release tablets is to mix the prescription amount of atenolol, HPMCK15M, Eudragit RSPO, high viscosity PEO, and lactose monohydrate in equal amounts. Then add 1% magnesium stearate and micro-powdered silica gel as lubricants, and adopt the powder direct pressing process to obtain atenolol pH-independent sustained-release tablets.
Embodiment 3
[0061] Atenolol pH-independent sustained-release tablet unit prescription (1000 tablets, 25mg per tablet). It contains the following components and weight:
[0062] Atenolol 25g
[0063] HPMC K15M 80g
[0064] Utraki RSPO 75g
[0065] PEO(POLYOX) 25g
[0066] Lactose 42.5g
[0067] Micronized silica gel and magnesium stearate 2.5g
[0068] The preparation method of the above-mentioned atenolol pH-independent sustained-release tablets is to mix the prescription amount of atenolol, HPMCK15M, Eudragit RSPO, high viscosity PEO, and lactose monohydrate in equal amounts. Then add 1% magnesium stearate and micro-powdered silica gel as lubricants, and adopt the powder direct pressing process to obtain atenolol pH-independent sustained-release tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com