Function and application of IRF3 (Interferon Regulatory Factor 3) to restenosis after stenting and carotid endarterectomy
A technology for restenosis and vascular stenosis, which can be applied to medical preparations containing active ingredients, peptide/protein ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Mouse Vascular Injury Model (VI) Obtained
[0030] 1. Grouping of experimental animals: WT and IRF3-KO mice aged 8-10 weeks and weighing 24-27g were used and divided into four groups: WT vascular injury group; WT sham operation group; IRF3-KO vascular injury group; IRF3 - KO sham operation group, 60 mice in each group. Twenty mice in each group were sacrificed 7 days, 14 days, and 28 days after the operation, and blood vessels in the injured segment were collected for analysis.
[0031] 2. Operation procedure of mouse vascular injury model:
[0032] 1) Accurately weigh the body weight (g) of the mouse in dynamic mode with an electronic balance, accurately prepare 3% pentobarbital sodium solution with double distilled water, shake gently to dissolve it fully, and use 80mg / kg body weight dose to calculate Accurately extract the corresponding volume of pentobarbital sodium solution with a 1mL syringe, and intraperitoneally inject the anesthetized mouse. After t...
Embodiment 2
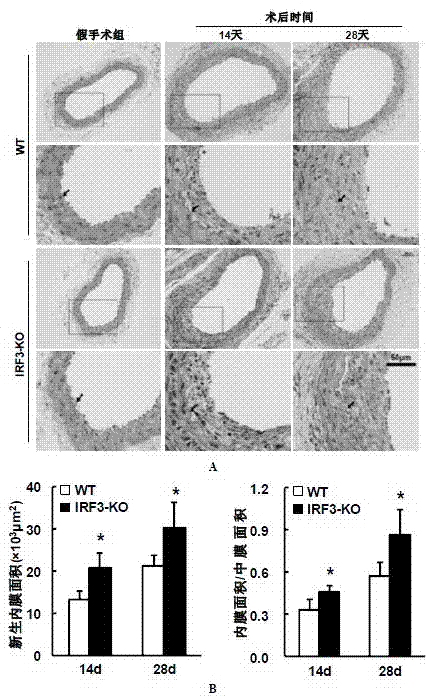
[0037] Example 2 Determination of Intimal Neogenesis in Vascular Injury Model (VI) Mice
[0038] 1. Mice Harvesting
[0039] 1) Anesthetize the mouse, cut the heart and let the blood out.
[0040]2) Cut the carotid artery from the proximal bifurcation of the carotid artery, take 0.5-0.6cm long, and keep the external carotid artery knot.
[0041] 3) Put the carotid artery in PBS, and gently drain the residual blood in the lumen with micro forceps.
[0042] 4) Put the blood vessel into a 1.5mL EP tube filled with 1mL 4% paraformaldehyde for fixation.
[0043] 2. Pathological detection
[0044] 2.1 Preparation of paraffin specimen slices
[0045] Paraffin specimen sections are prepared by professional pathological staff in the laboratory. The main operating procedures include trimming the heart → processing the embedding frame → washing with running water → dehydrating → transparent → dipping in wax → embedding → sectioning (3 μm) → spreading → drying or baking Backup.
[0...
Embodiment 3
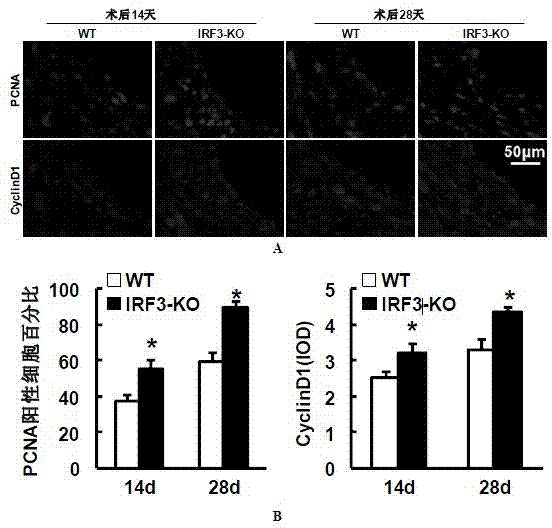
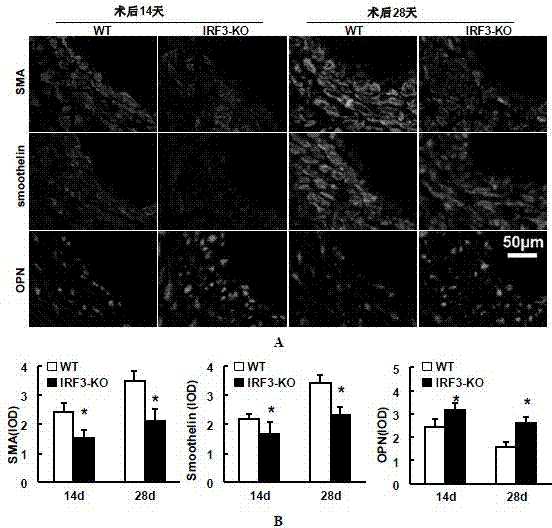
[0053] Example 3 Detection of proliferation level of blood vessel wall cells
[0054] The expressions of proliferating cell nuclear antigen (PCNA) and cell cycle protein (Cyclin D1) were detected by immunofluorescence staining. Required primary antibody information: PCNA (#2586; 1:100; mouse; Cell Signaling Technology), cyclin D1 (#2978; 1:25; rabbit; Cell Signaling Technology); required secondary antibody information: Alexa Fluor 568-conjugated goat anti-rabbit IgG (A11011; Invitrogen, Carlsbad, CA), Alexa Fluor 568-conjugated goat anti-mouse IgG (A11004; Invitrogen, Carlsbad, 150 d, CA).
[0055] The main steps are:
[0056] 1) Baked slices: put the paraffin slices in the oven for more than 30 minutes.
[0057] 2) Dewaxing: Xylene 5min×3.
[0058] 3) Hydration: 100% ethanol 5min×2; 95% ethanol 5min; 70% ethanol 5min; ddH 2 O dipping for 5min×2.
[0059] 4) Citrate tissue antigen repair (high pressure repair): Take a certain amount of pH6.0 citrate antigen repair working...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com