Star-structured polyethylene and preparation method thereof
A polyethylene and star-shaped structure technology, applied in the field of star-shaped polyethylene and its preparation, can solve the problems of poor practicability, limited, complex synthesis routes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
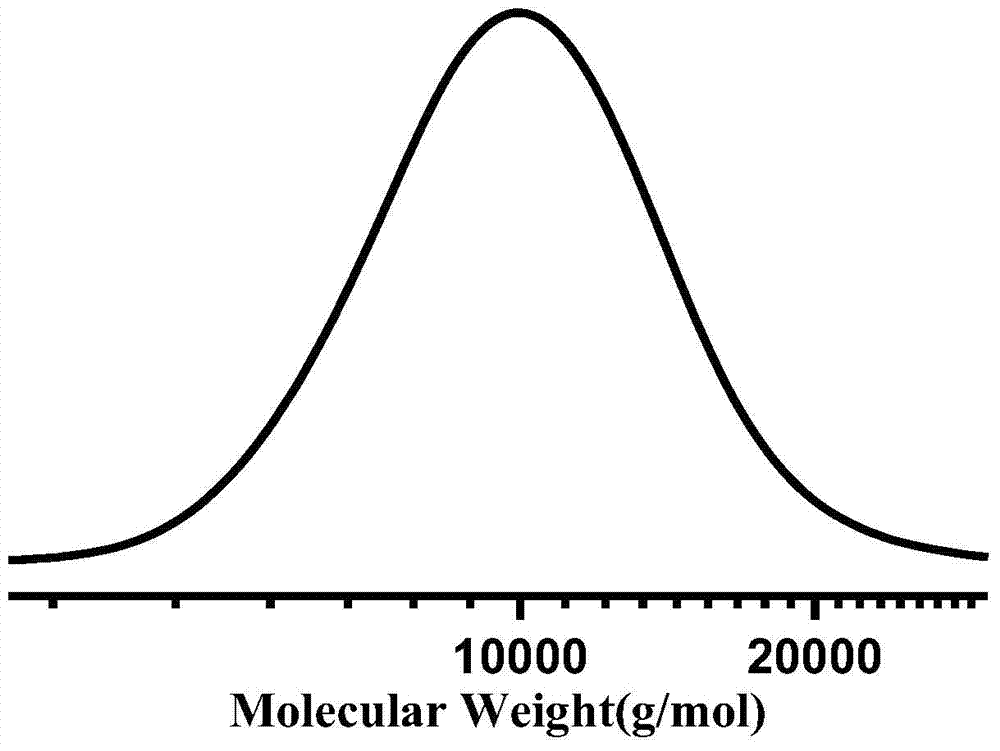
[0093] In a 250ml two-necked bottle, add 5g terminal double bond polyethylene (number average molecular weight Mn=2000g / mol, polydispersity index PDI=2.4), vacuum dry at 40°C for 1 hour, fill with nitrogen, add 100ml toluene, and heat up to 110°C , stir to fully dissolve. 0.3 g of dioctanoyl peroxide and 6 g of (3-mercaptopropyl)trimethoxysilane were added under a nitrogen atmosphere. Continue to stir and react for 12 hours, precipitate the polymer with a large amount of methanol, filter, wash with methanol repeatedly at 50°C and dry in vacuum for 12 hours to obtain trimethoxysilane-terminated polyethylene.
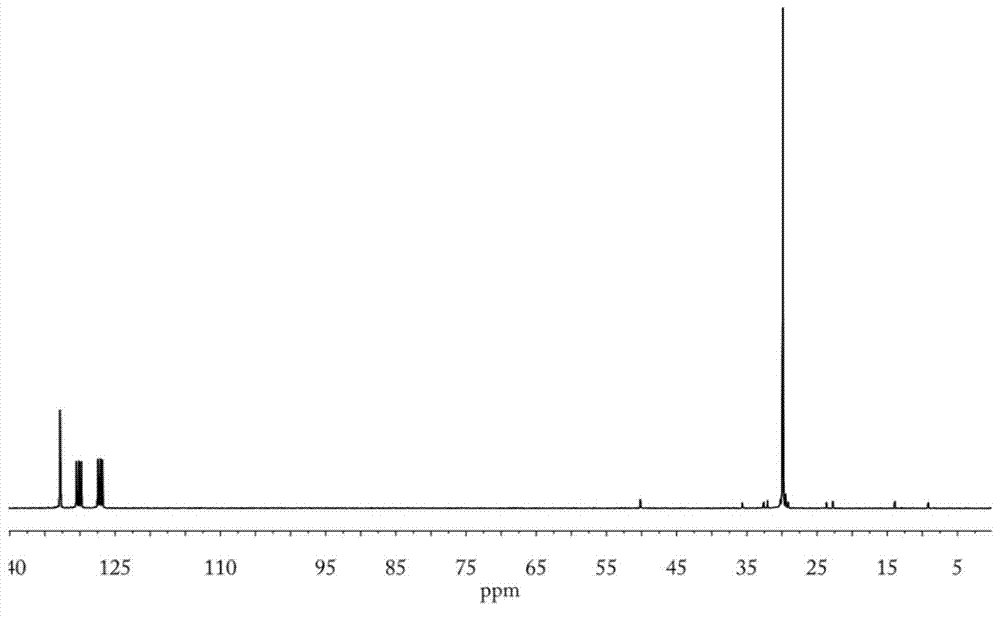
[0094] 1 H NMR and 13 C NMR characterization confirmed that the polyethylene terminal unsaturated double bond reaction was complete, and the selectivity of trimethoxysilane-capped polyethylene terminal group was higher than 90%.
Embodiment 2
[0096] In a 250ml two-necked bottle, add 5g terminal double bond polyethylene (number average molecular weight Mn=600g / mol, polydispersity index PDI=1.3), vacuum dry at 40°C for 1 hour, fill with nitrogen, add 100ml ethylbenzene, heat up to 80 ℃, stir to fully dissolve. 1.0 g of azobisisobutyronitrile and 10 g of (5-mercaptopentyl)triethoxysilane were added under a nitrogen atmosphere. Continue to stir and react for 7 hours, precipitate the polymer with a large amount of methanol, filter, wash with methanol repeatedly at 50°C and dry in vacuum for 12 hours to obtain triethoxysilane-capped polyethylene.
[0097] 1 H NMR and 13 C NMR characterization confirmed that the polyethylene terminal unsaturated double bond reaction was complete, and the selectivity of triethoxysilane-capped polyethylene terminal group was higher than 90%.
Embodiment 3
[0099] In a 100ml two-necked bottle, add 4g of epoxy group-terminated polyethylene (number-average molecular weight Mn=1500g / mol, polydispersity index PDI=3.5), vacuum dry at 40°C for 1 hour, fill with nitrogen, add 50ml of chlorobenzene and 10g (3-aminopropyl)triethoxysilane, heated up to 100°C, stirred to fully dissolve. After reacting for 10 hours, the polymer was precipitated with a large amount of methanol, filtered, washed with methanol repeatedly at 50° C. and dried in vacuum for 12 hours to obtain triethoxysilane-capped polyethylene.
[0100] 1 H NMR and 13 C NMR characterization confirmed that the polyethylene terminal hydroxyl groups were completely converted, and the selectivity of triethoxysilane-capped polyethylene terminal groups was higher than 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com