Method for preparing high-yield and high-purity agomelatine crystal I
An agomelatine, high-purity technology, applied in the field of medicinal chemistry synthesis, can solve the problems of poor stability, low crystal form purity, poor reproducibility, etc., to improve yield and yield, reduce reagent cost and energy consumption , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
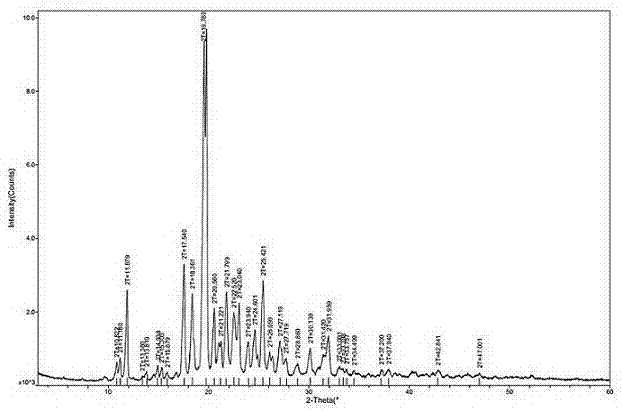
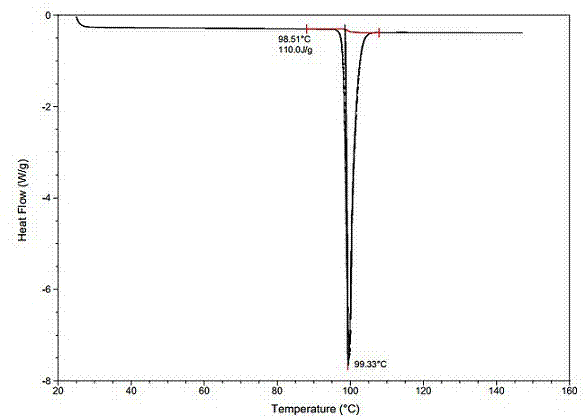
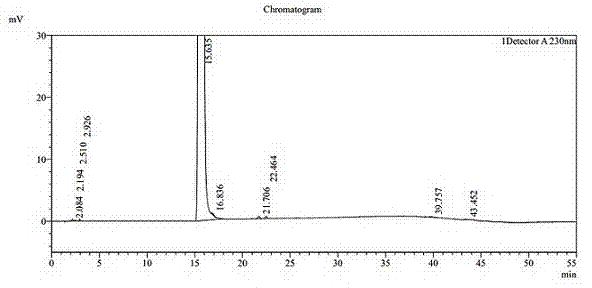
[0036] Dissolve 20g of agomelatine in a mixed solvent of 50mL of DMF and 25mL of purified water (the dissolution reaction temperature is 50°C); suction filtration under a pressure of -0.09MPa; slowly add the filtrate dropwise to 400mL while stirring at 180r / min Frozen purified water at 1°C, add dropwise for 2 hours; stir at constant temperature at 1°C for 1 hour after dropping; filter under -0.09MPa pressure; rinse with 40mL purified water, and put the obtained filter cake into a vacuum oven with 100g of phosphorus pentoxide After heat preservation at 40°C and drying for 72 hours, 18.92 g of white solid powder was obtained with a melting point of 98.3°C to 99.3°C and a yield of 94.6%. The white solid powder was verified as agomelatine crystal form I, with a purity of 99.96% (HPLC content), and a single impurity of 9ppm. Its HPLC test result is shown in Table 1 and attached image 3 , see the attached DSC test results figure 2 , XRPD test results are shown in Table 2 and att...
Embodiment 2
[0042] Dissolve agomelatine 16g in a mixed solvent of DMF 40mL and purified water 20mL (dissolution reaction temperature is 45°C); filter under -0.09MPa pressure; slowly add the filtrate dropwise to 320mL with stirring at 190r / min Frozen and purified water at 1°C, dropwise for 110min; after dropping, stir at 1°C for 1h; filter under -0.09MPa pressure; rinse with 30mL of purified water, and put the obtained filter cake into a vacuum oven with 80g of phosphorus pentoxide After heat preservation at 45°C and drying for 48 hours, 18.80 g of white solid powder was obtained with a melting point of 98.2°C to 99.1°C and a yield of 94%. The white solid powder was verified as agomelatine crystal form I, with a purity of 99.94% (HPLC content), and a single impurity of 9ppm. Its XRPD test result is shown in Table 3 and attached Figure 4 .
[0043]
[0044] The XRPD test data of table 3 embodiment two
[0045]
Embodiment 3
[0047]Dissolve 8g of agomelatine in a mixed solvent of DMF 20mL and purified water 8mL (dissolution reaction temperature is 50°C); filter under -0.09MPa pressure; slowly add the filtrate dropwise to 160mL with stirring at 180r / min Frozen purified water at 1°C, dropwise for 110 minutes; after dropping, stir at 0°C for 1 hour; filter under -0.09MPa pressure; rinse with 20mL of purified water, and put the obtained filter cake into a vacuum oven with 50g of phosphorus pentoxide After heat preservation at 45°C and drying for 72 hours, 18.84 g of white solid powder was obtained, with a melting point of 98.3°C to 99.3°C and a yield of 94.2%. The white solid powder was verified as agomelatine crystal form I, with a purity of 99.95% (HPLC content), and a single impurity of 8ppm. Its XRPD test result is shown in Table 4 and attached Figure 5 .
[0048] The XRPD test data of table 4 embodiment three
[0049]
[0050] The present invention has mild reaction conditions, simple opera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com