Fluoroborate pyrrole photosensitizer and synthetic method thereof
A technology of boron pyrrole photosensitizer and boron fluoride dipyrrole, which is applied in the field of new boron pyrrole photosensitizer and its synthesis, can solve the problems of high price and high cost, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
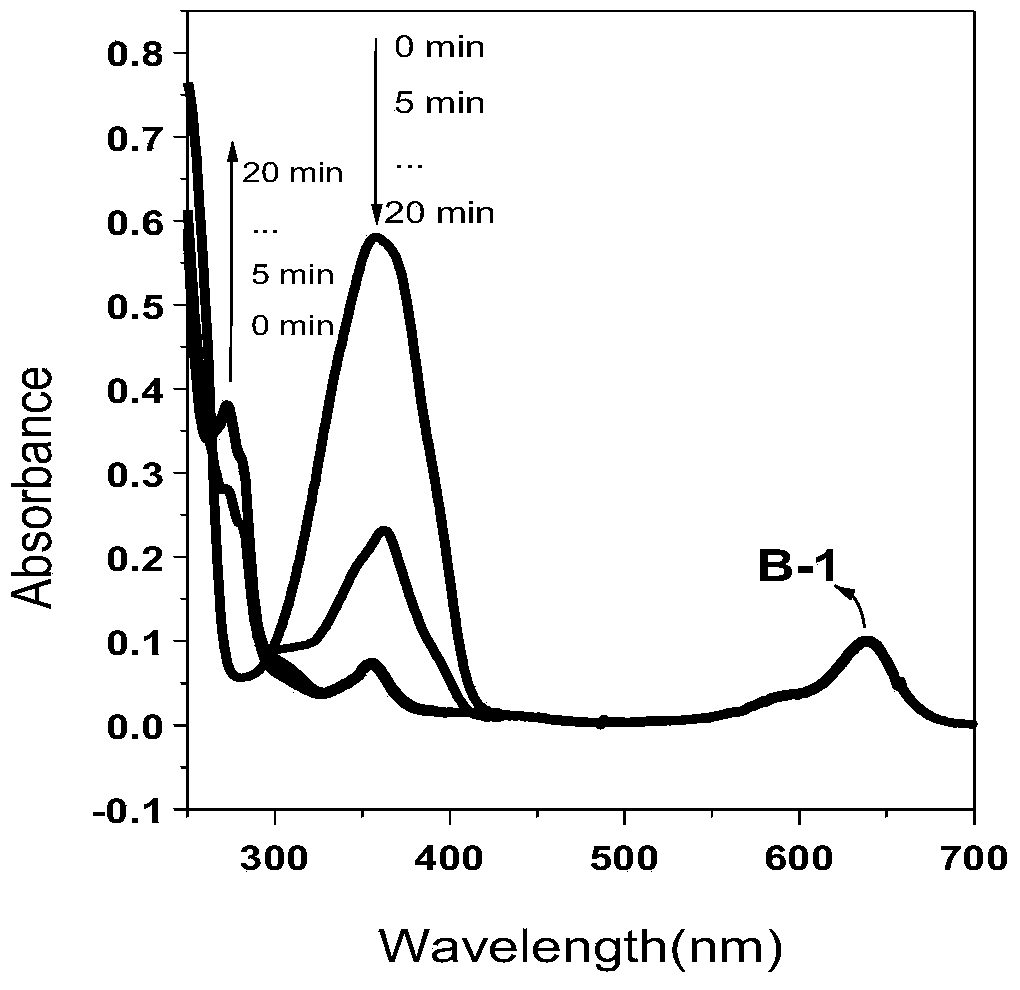
[0048] Example 1: Microwave heating synthesis method of β-position iodoboron fluoride dipyrrole 3,5-position phenenyl derivatives
[0049] Dissolve β-iodoboron fluoride dipyrrole (50mg, 0.09mmol) and benzaldehyde (38mg, 0.36mmol) in 5ml N'N-dimethylformamide, glacial acetic acid (3 drops), piperidine (3 drop) was quickly added dropwise into the reaction solution, microwaved at 150°C for 15 minutes under an argon atmosphere, the product was washed with water to remove the solvent, filtered with suction, the filter cake was dissolved in dichloromethane, and the solvent was evaporated under reduced pressure to obtain a crude product. Purify by column chromatography (developer: dichloromethane) to obtain dark purple powder (40mg, 0.054mmol, 60%). 1 H NMR (400MHz, CDCl 3 ):δ=8.18-8.14(d,J=16.0Hz,2H),7.73(s,1H),7.69-7.66(m,5H),7.55-7.52(m,3H),7.44-7.41(m,4H ),7.37-7.33(d,J=16.0Hz,2H),7.31-7.29(m,2H),1.46ppm(s,6H).MALDI-HRMS calcd[C 33 h 25 BF 2 N 2 I 2 ] + m / z752.0168, found...
Embodiment 2
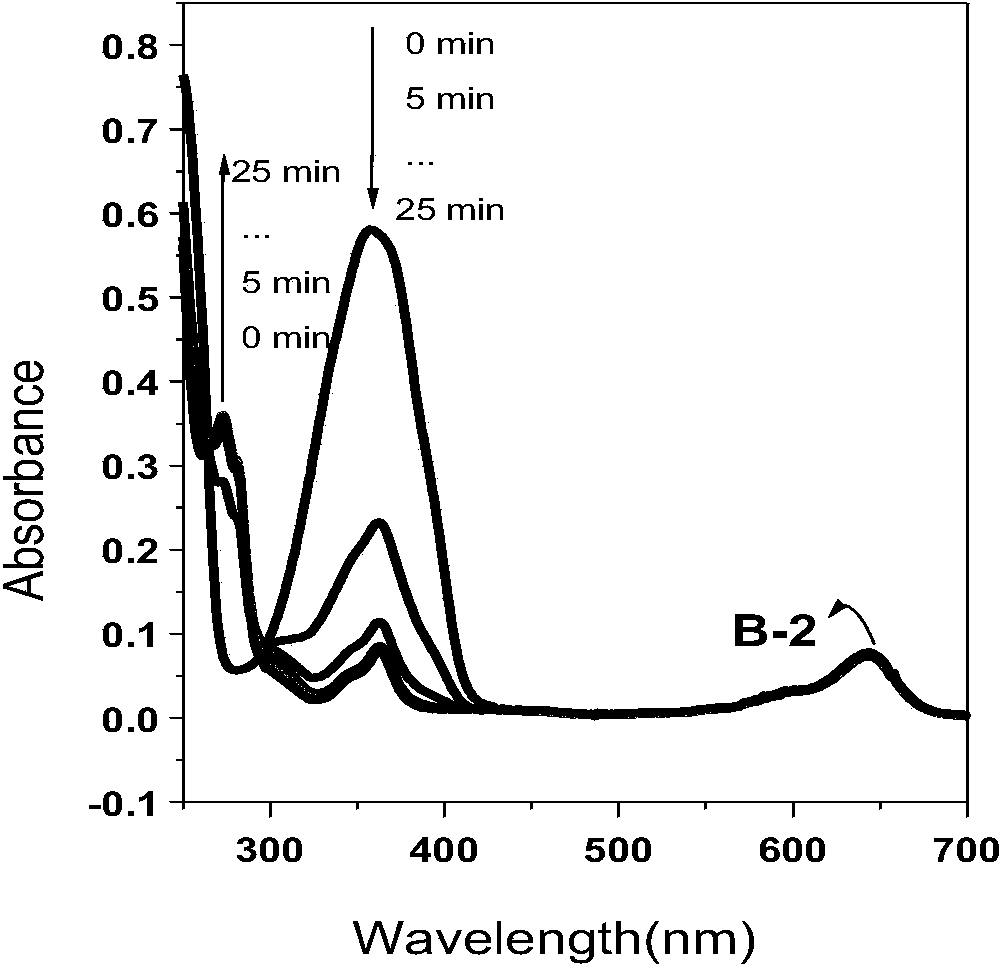
[0050] Example 2: Microwave heating synthesis method of β-position iodoboron fluoride dipyrrole 3,5-position p-cyanophenenyl derivatives
[0051] Dissolve β-iodoboron fluoride dipyrrole (50mg, 0.09mmol) and p-cyanobenzaldehyde (47mg, 0.36mmol) in 5ml N'N-dimethylformamide, glacial acetic acid (3 drops), piperazine Pyridine (3 drops) was quickly added dropwise to the reaction solution, microwaved at 150°C for 15 minutes under an argon atmosphere, the product was washed with water to remove the solvent, filtered with suction, the filter cake was dissolved in dichloromethane, and the solvent was evaporated under reduced pressure to obtain the crude product . Purify by column chromatography (developing solvent: dichloromethane) to give dark purple powder (36mg, 0.045mmol, 50%). 1 H NMR (400MHz, CDCl 3 ):δ=8.17-8.14(d,J=12.0Hz,1H),8.03-8.01(d,J=8.0Hz,1H),7.88-7.86(d,J=8.0Hz,1H),7.79(s, 1H),7.75-7.73(m,7H),7.60-7.59(d,J=4.0Hz,2H),7.56-7.54(m,2H),7.33-7.31(d,J=8.0Hz,2H),1.50 (s,6...
Embodiment 3
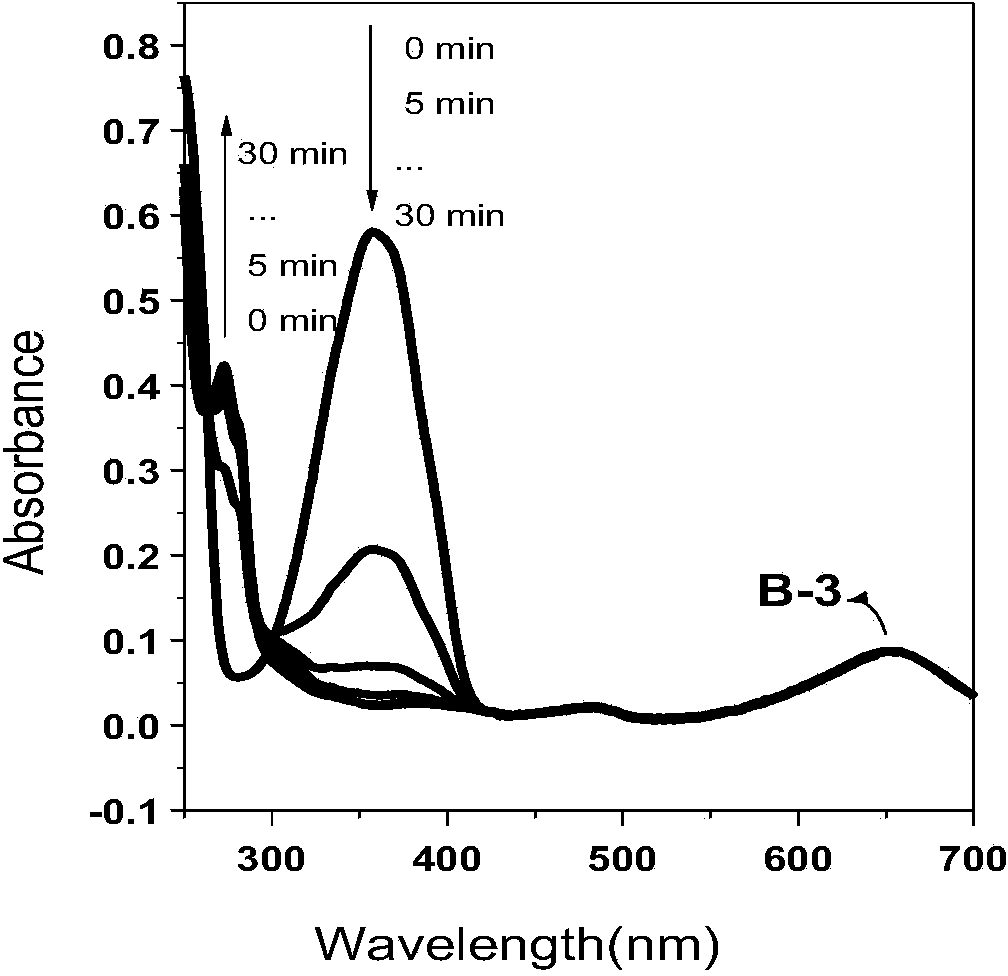
[0052] Example 3: Microwave heating synthesis method of β-position iodoboron fluoride dipyrrole 3,5-position p-nitrophenenyl derivatives
[0053] Dissolve β-iodoboronium fluoride dipyrrole (50mg, 0.09mmol) and p-nitrobenzaldehyde (54mg, 0.36mmol) in 5ml N'N-dimethylformamide, glacial acetic acid (3 drops), piperazine Pyridine (3 drops) was quickly added dropwise to the reaction solution, microwaved at 150°C for 15 minutes under an argon atmosphere, the product was washed with water to remove the solvent, filtered with suction, the filter cake was dissolved in dichloromethane, and the solvent was evaporated under reduced pressure to obtain the crude product . Purify by column chromatography (developing solvent: dichloromethane) to obtain dark purple powder (38mg, 0.045mmol, 50%). 1 H NMR (400MHz, CDCl 3 ):δ=8.14-8.10(d,J=16Hz,4H),7.56-7.53(d,J=12Hz,4H),7.30-7.28(m,3H),7.21-7.18(d,J=12Hz,2H ),7.14-7.11(m,4H),1.88(s,6H).MALDI-HRMS calcd[C 33 h 23 BF 2 N 4 I 2 o 4 ] + m / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com