Thienopyrroledione-based micromolecular acceptor material as well as preparation method and application of thienopyrroledione-based micromolecular acceptor material
A small-molecule acceptor and diketopyrrole-type technology, which is applied in semiconductor/solid-state device manufacturing, electrical solid-state devices, semiconductor devices, etc., can solve problems affecting the absorption of active layer materials and affecting the photogenerated current of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
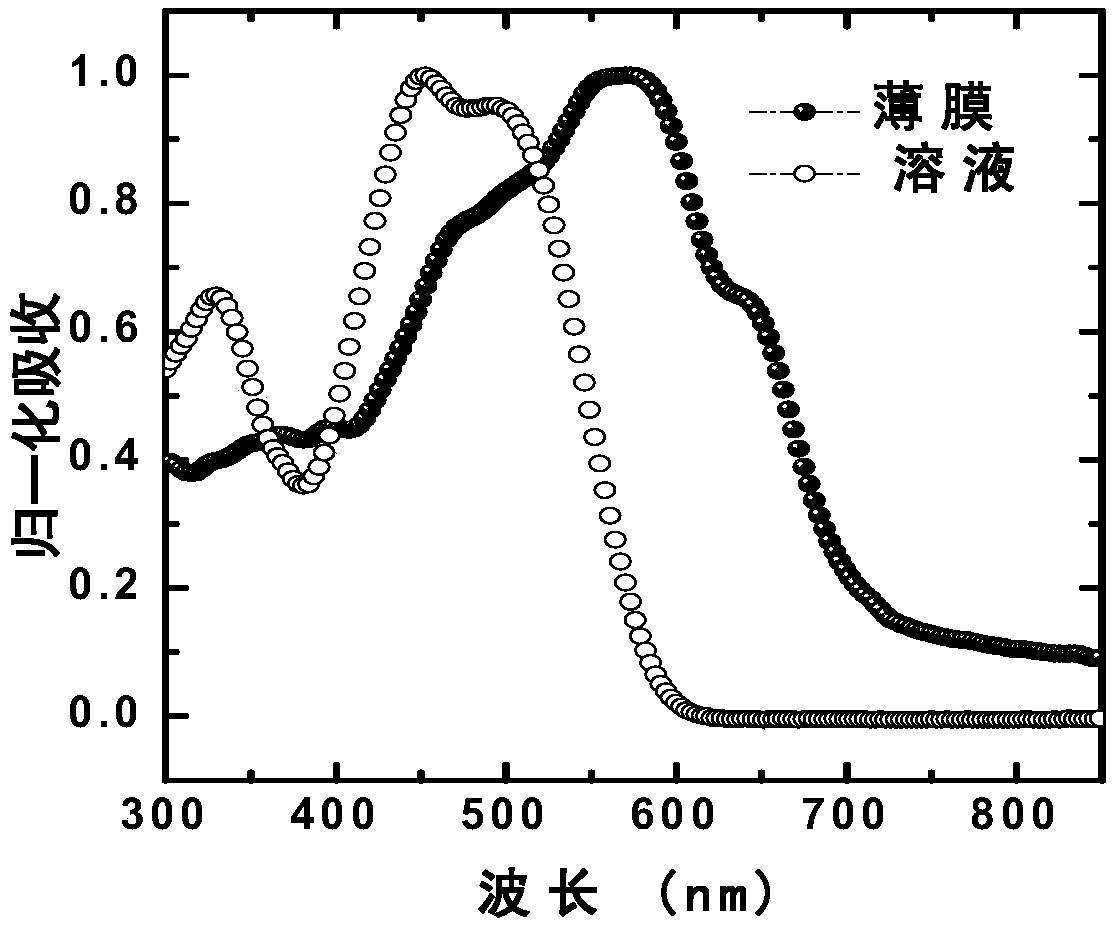
Image
Examples
Embodiment 1
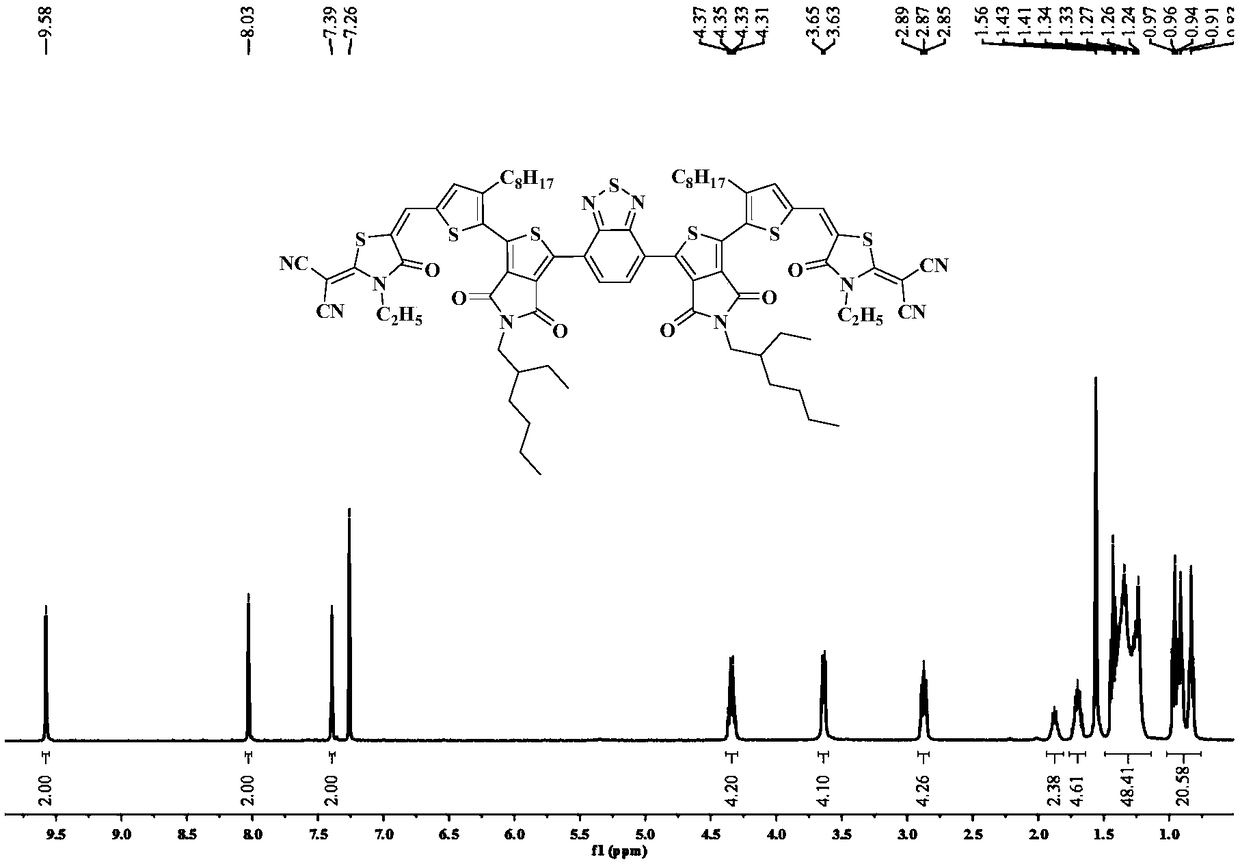
[0083] Thienopyrrole diketone (EHTPDThRCN) based on the present embodiment 2 The structural formula of BT small molecule acceptor material:
[0084]
[0085] Its synthetic steps are as follows:
[0086] Synthesis of Step 1 compound 4,7-dibromobenzo[c][1,2,5]thiadiazole
[0087]
[0088] Add benzo[c][1,2,5]thiadiazole (10g, 0.073mol) to 150mL hydrobromic acid solution (8.7mol / L hydrobromic acid solution), and slowly add it with a constant pressure dropping funnel Liquid bromine (25.66g, 0.1606mol), after the addition, the reaction was refluxed at 130°C for 12h, after cooling, slowly add sodium hydroxide solution under ice bath conditions, adjust the pH to about 7, then add dichloromethane and Extract with distilled water, dry the organic layer with anhydrous magnesium sulfate, filter, distill off the solvent under reduced pressure, and then separate with a silica gel column. The eluents are petroleum ether and dichloromethane to obtain 19.7 g of light yellow crystals, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
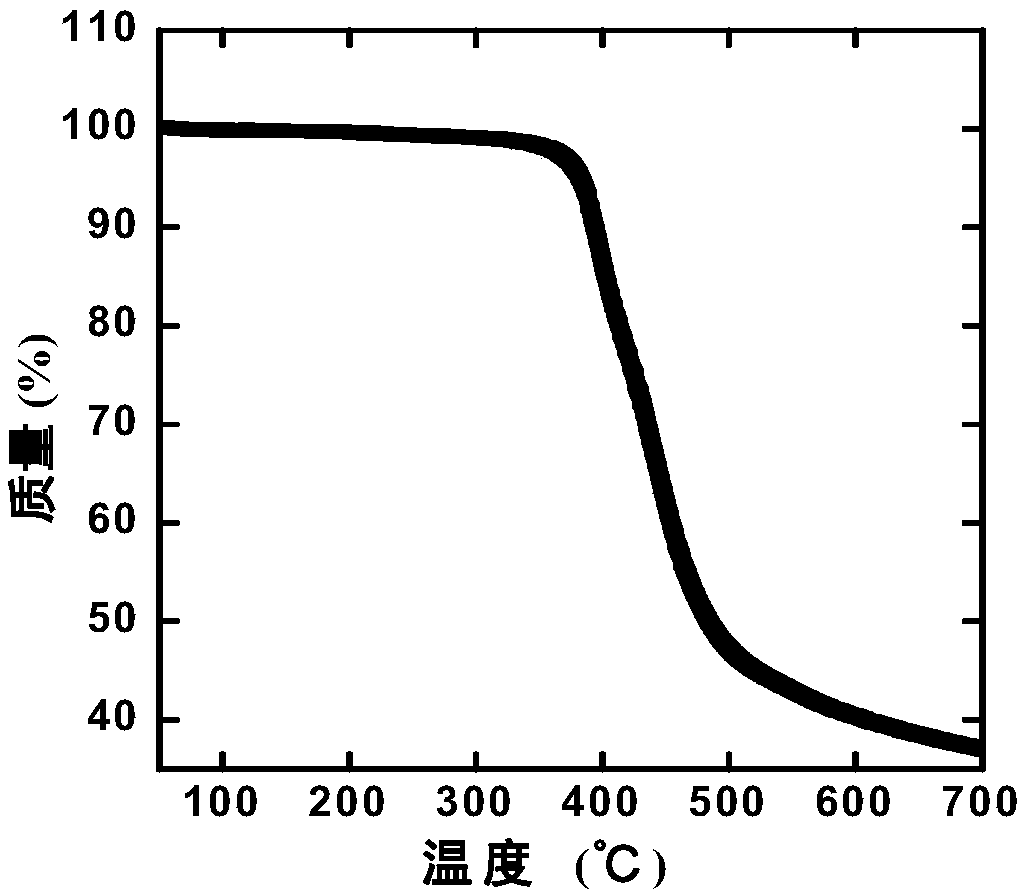
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com