Cefathiamidine compound
A cefathiamidine and compound technology, which is applied in the field of cefathiamidine compounds, can solve the problems such as the need to improve the crystal purity of cefathiamidine, and achieve the effects of safety, reliability, good stability and high stability in clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of cefathiamidine compound
[0034] 1. Prepare 5 L of saturated aqueous solution of cefathiamidine crude product;
[0035] 2. In a sound field with a frequency of 28KHz and an output power of 50W, under the condition of nitrogen filling, add 20L of a mixed solution of dichloroethylene and cyclohexane at 0°C while stirring, and the addition speed is 100ml / min. The volume ratio of ethylene and cyclohexane is 1:3; after adding the mixed solution, adjust the frequency of the sound field to 18KHz, cool down to 0°C, and the cooling rate is 1°C / hour; grow crystals for 6 hours, remove the sound field after the crystals are precipitated , washed and dried to obtain the cefathiamidine compound.
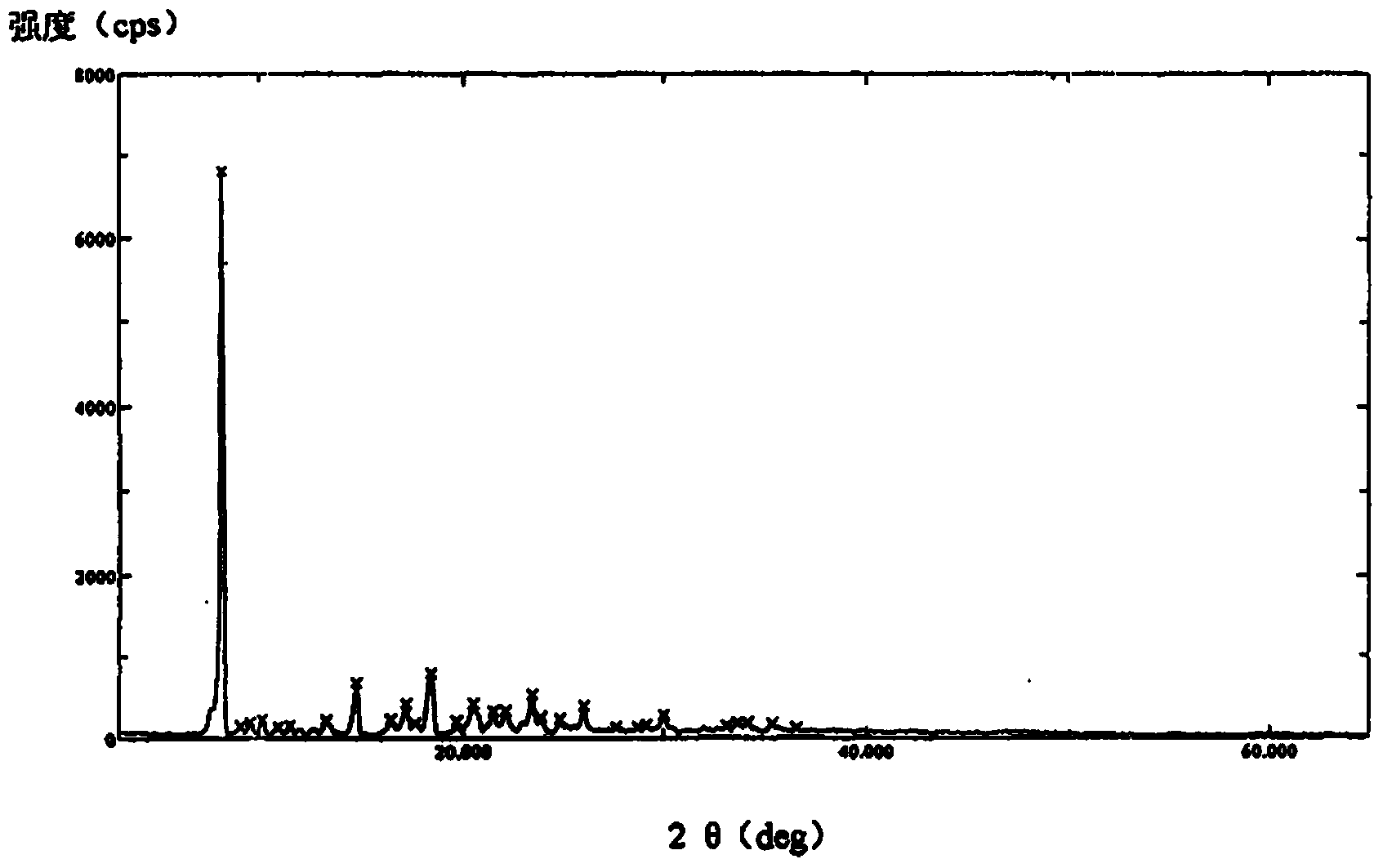
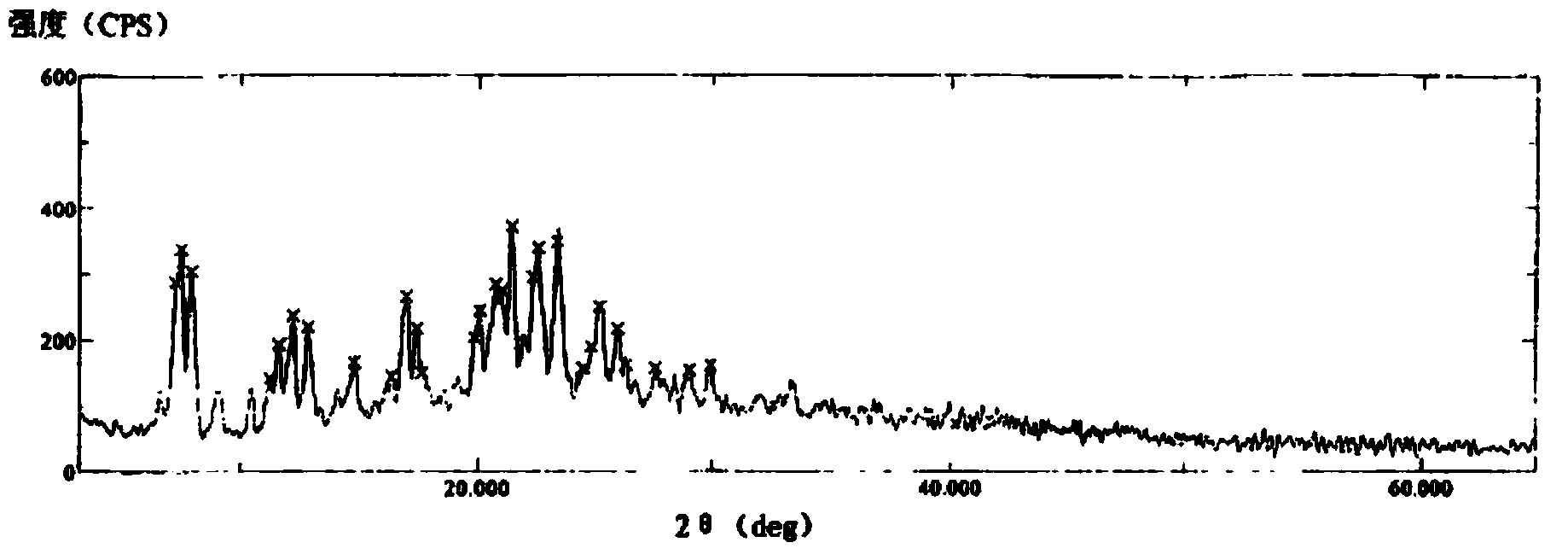
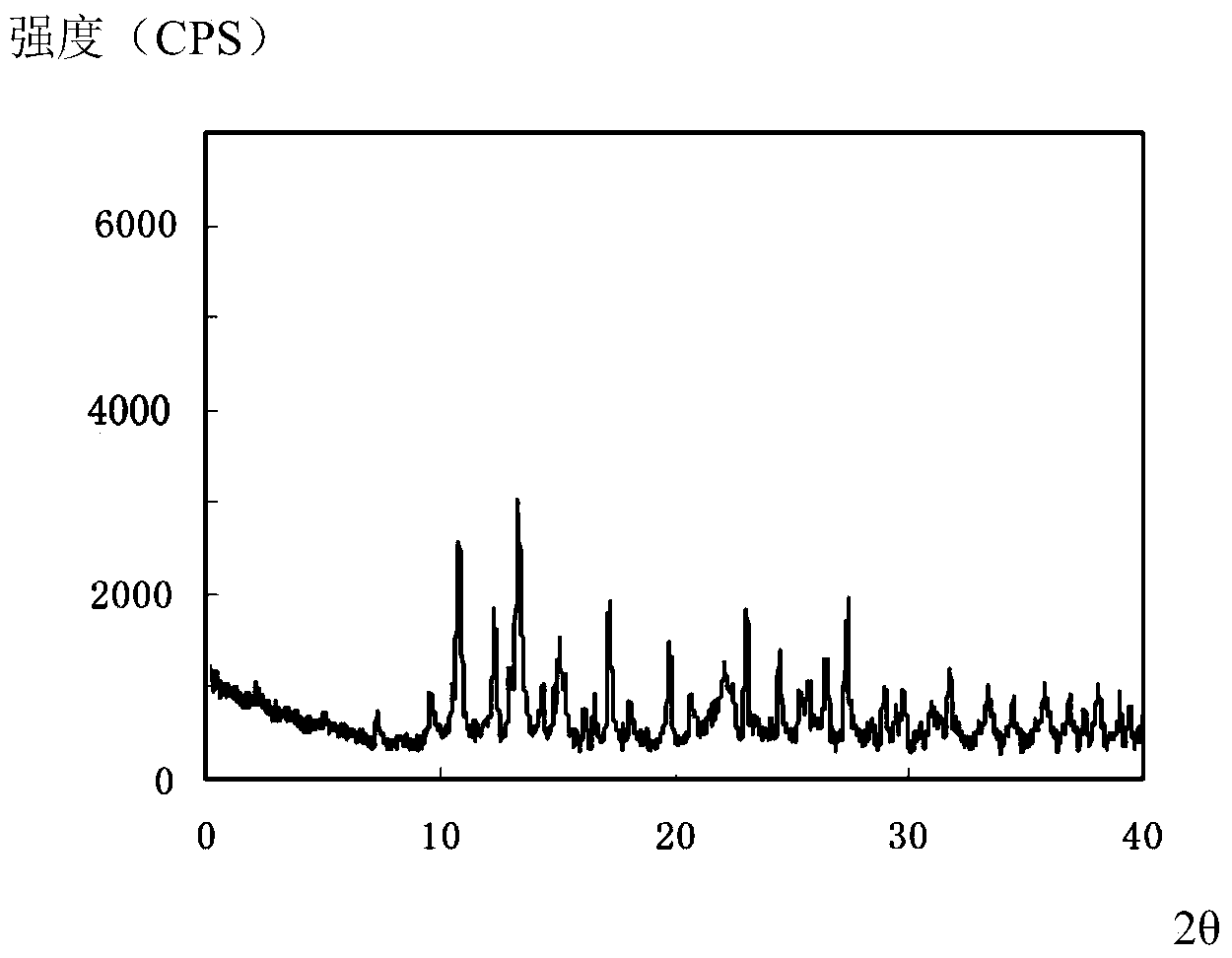
[0036] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 95.6%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows image 3 Shown; Observation by s...
Embodiment 2
[0037] Embodiment 2: the preparation of cefathiamidine compound
[0038] 1. Prepare 5 L of saturated aqueous solution of cefathiamidine crude product;
[0039] 2. In a sound field with a frequency of 25KHz and an output power of 40W, under the condition of nitrogen filling, add 15L of a mixed solution of dichloroethylene and cyclohexane at 5°C while stirring, and the addition speed is 75ml / min. The volume ratio of ethylene and cyclohexane is 1:4; after adding the mixed solution, adjust the frequency of the sound field to 15KHz, cool down to 0°C, and the cooling rate is 1°C / hour, grow crystals for 6 hours, remove the sound field after the crystals are precipitated , washed and dried to obtain the cefathiamidine compound.
[0040] The compound crystal is detected by high-performance liquid chromatography, and the purity is 99.96%, and the yield is 95.6%; the X-ray powder diffraction pattern obtained by using Cu-Kα ray measurement is as follows image 3 Shown; Observation by sc...
experiment example 1
[0041] Experimental example 1: Influencing factor experiment
[0042] 1. High temperature test
[0043] Take three batches 101, 102, and 103 of the cefathiamidine crystalline compound prepared in Example 1, put them in simulated market packaging, place them in a sealed clean container, and place them at a temperature of 40±2°C for 10 days. Sampling was carried out on day 0, tested according to key stability inspection items, and the test results were compared with those on day 0.
[0044] 2. High humidity test
[0045] Take three batches 101, 102, and 103 of the cefathiamidine crystalline compound prepared in Example 1, put them in simulated market packaging, put them in a sealed clean container, and place them at 25±2°C for 10 days at a relative humidity of 90%±5%. , samples were taken on the 5th and 10th days, tested according to the key investigation items of stability, and the test results were compared with those on day 0.
[0046] 3. Strong light irradiation test
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com