2,4,6-trisubstituted pyrimidine compounds containing 1,2,3-triazole, preparation method and application thereof
A compound, tri-substituted technology, applied in the application field of anti-tumor drug lead compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] general formula( II ) is prepared with reference to the following documents:
[0060] H.I. Skulnick, J.H. Ludens, M.G. Wendling. Journal of Medicinal Chemistry , 1986 , 29, 1499-1504.
[0061] general formula( IV ) is prepared with reference to the following documents:
[0062] (a) Ina Wilkening.; Giuseppe del Signore.; C. P. R. Hackenberger. Chem. Commun. 2011 , 47, 349-351. (b) Mingyu Hu.; Junqi Li.; ShaoQ Yao. Org. Lett , 2008 , 10, 5529-5531.
Embodiment
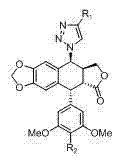
[0063] Example 1 general formula( II ), R 3 =H, the derivative of ( II-1 ) preparation
[0064] Add ethyl cyanoacetate (2.262g, 20mmol) and sodium hydroxide (1.200g, 30mmol) into the ethanol solution, under reflux conditions, react for a period of time, then add thiourea (2.284g, 30mmol) and benzaldehyde (3.184 g, 30mmol) was added into the reaction system, stirred and reacted, followed by TLC detection. After the reaction, filter with suction and recrystallize to obtain the pure product.
[0065] Example 2 general formula( III ), R 3 = H, a derivative of ( III-1 ) preparation
[0066] Add propyne bromide (3.569g, 30mmol) dropwise into a solution of II-1 (2.674g, 10mmol) in 1,4-dioxane, heat and stir to react. Monitor the reaction process with TLC until the reaction is complete; then directly dropwise add phosphorus oxychloride (4.600g, 30mmol) in the reaction system, after the reaction is complete, pour it into ice water, stir, solids are precipitated, and su...
Embodiment 32
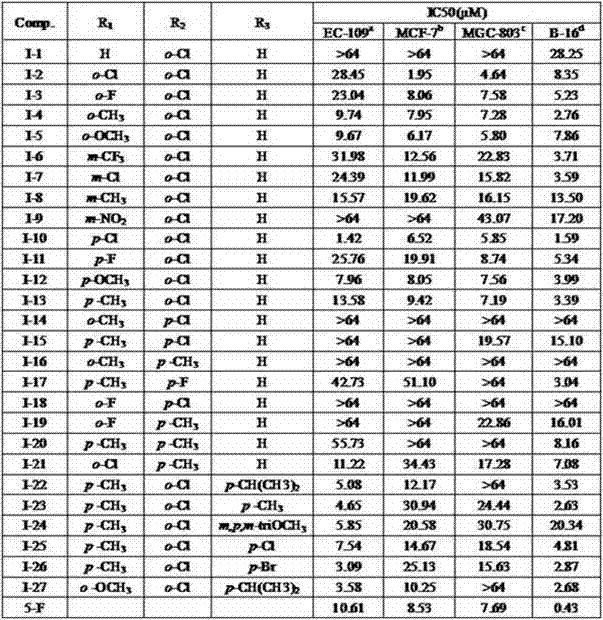
[0139] Example 32 The antitumor activity assay of above-mentioned compound:
[0140] 1. Experimental method:
[0141] The compounds used in the screening are all synthesized and purified by the present invention; sample stock solution: weigh 3-5 mg of the sample and place it in a 1.5 mL EP tube, and then use DMSO to prepare a concentration of 128×10 3 μg / mL solution, stored at 4 °C, and diluted with medium according to the required concentration during the experiment.
[0142] 2. Primary screening
[0143] Take the cells in the logarithmic growth phase, digest and count them, adjust the cell density with medium, inoculate 4000-5000 cells / well into a 96-well plate, 150 μL per well, culture for 24 h, discard the medium, add Drugs (50 μg / mL, 100 μg / mL) diluted with culture medium were used, and 6 replicate wells were set up for each concentration, and a blank control group and a negative control group were also set up. After 72 h of drug action, add 20 μL MTT to ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com