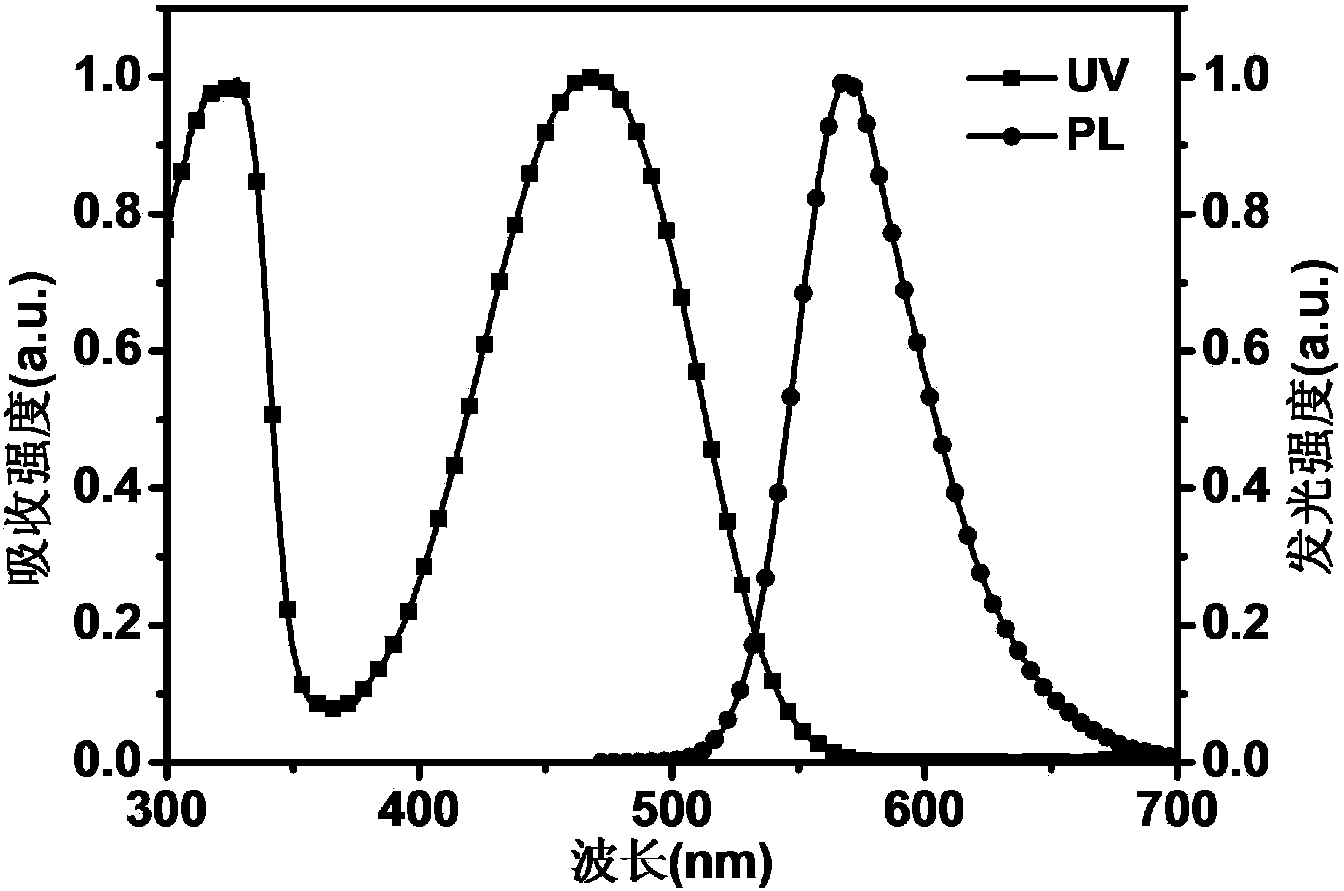
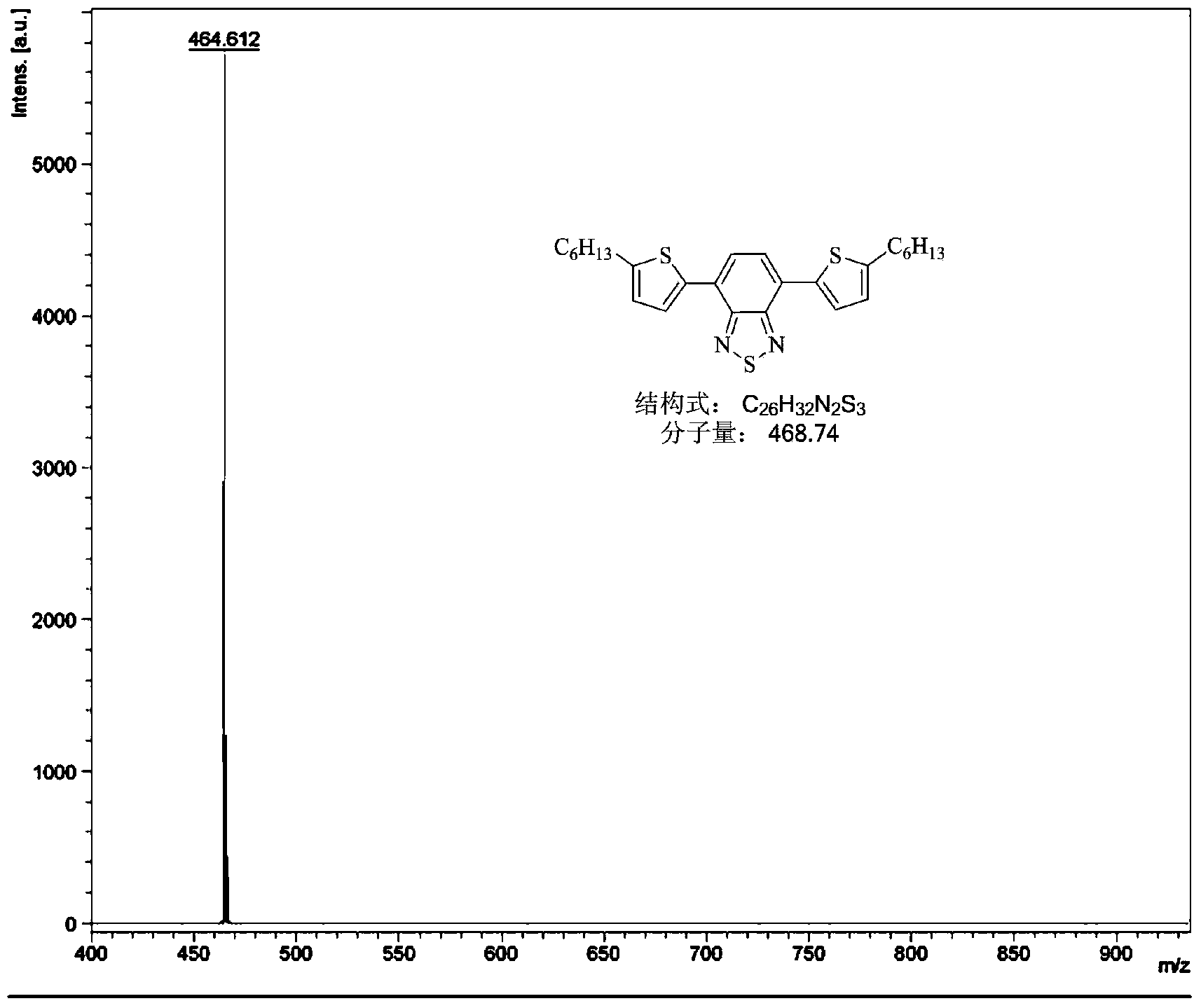
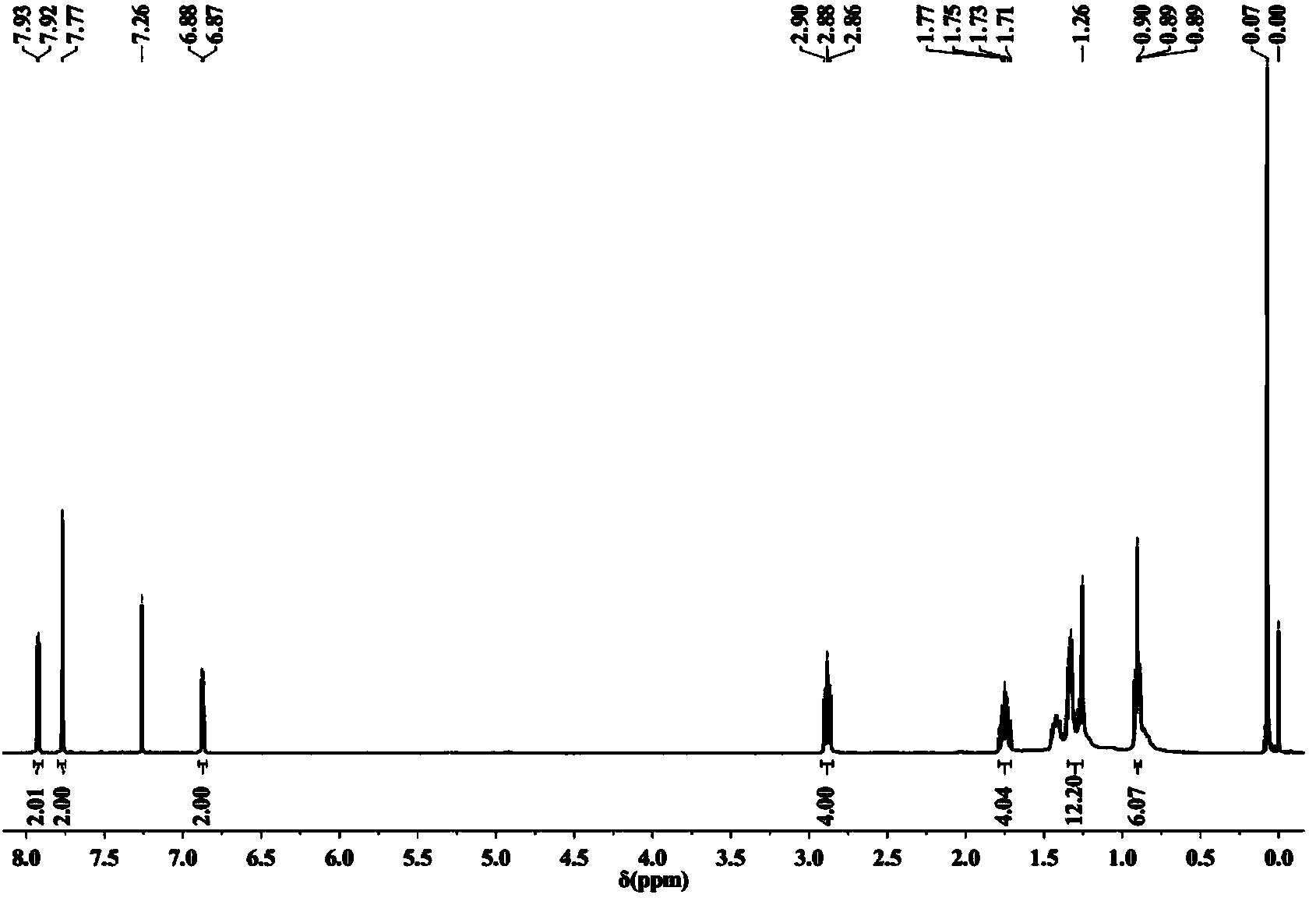
Narrow-energy-gap organic solar cell material and preparation method thereof
A solar cell and narrow bandgap technology, applied in organic chemistry, circuits, photovoltaic power generation, etc., can solve the problems of limiting the photoelectric conversion efficiency of solar cell devices, narrow spectral absorption range, etc., and achieve broadened spectral response range and wide solar spectral response range , The effect of improving the carrier transport characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] Target compound P1
[0023] According to the reaction scheme, compound 1 and liquid bromine were mixed in hydrobromic acid solvent and reacted at 110°C to obtain compound 2. Compound 3 was dissolved in freshly distilled tetrahydrofuran solvent, reacted with butyllithium at -78°C, and then bromohexane was added to obtain compound 4. 4 was dissolved in freshly distilled tetrahydrofuran solvent, reacted with n-butyllithium at -78°C, and isopropanol pinacol borate was added to give 5. Combine 5 and 2 at 2mol / mL K 2 CO 3 Mix in solution and toluene solvent, in tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 ) as a catalyst, the Suzuki coupling reaction occurred to obtain the target compound P1.
[0024] 【Reaction Roadmap】
[0025]
Embodiment 2
[0027]
[0028] Target compound P2
[0029] According to the reaction scheme, compound 1 and liquid bromine were mixed in hydrobromic acid solvent and reacted at 110°C to obtain compound 2. Compound 3 was dissolved in freshly distilled tetrahydrofuran solvent, reacted with butyllithium at -78°C, and then brominated n-octane was added to obtain compound 4. 4 was dissolved in freshly distilled tetrahydrofuran solvent, reacted with n-butyllithium at -78°C, and isopropanol pinacol borate was added to give 5. Combine 5 and 2 at 2mol / mL K 2 CO 3 Mix in solution and toluene solvent, in tetrakistriphenylphosphine palladium (Pd(PPh 3 ) 4 ) as a catalyst, the Suzuki coupling reaction occurred to obtain the target compound P2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com