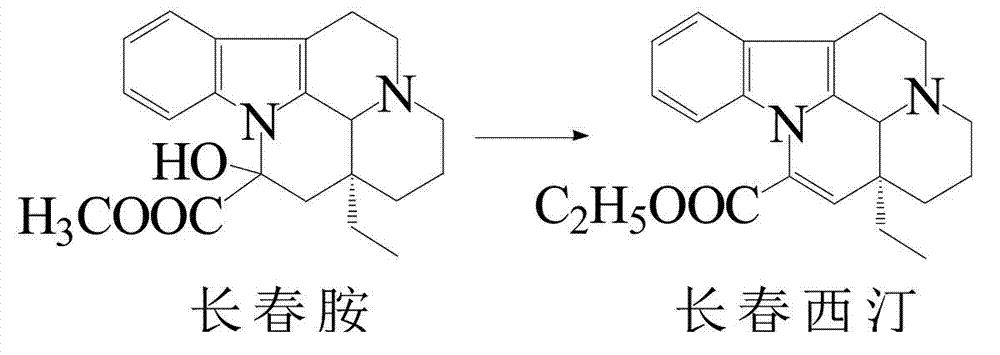
New synthetic process for vinpocetine semisynthesis
A technology of vinpocetine and vincamine, applied in the field of vinpocetine, which can solve the problems of lengthy steps, many by-products, and high price of vincamine, and achieve the effects of simple equipment, large economic and social benefits, and green production procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Synthesis of Vinpocetine
[0013] In a 500 L reactor, add 200 L of absolute ethanol as a solvent, 50 kg of vincamine as a reaction raw material, and 3.0 kg of sodium ethoxide as a catalyst in the reactor to make the pH of the system around 14, slowly raise the temperature to 70°C, and react for 3 h. Thin-layer chromatography monitors the complete reaction of the raw material vincamine, which is regarded as the completion of the hydrolysis reaction, and the generated product is a mixture of vincine and dehydrovincine. Continue in the same reaction kettle, stir and lower the temperature below 10°C, add glacial acetic acid to adjust the pH value to about 12, then raise the temperature to reflux, react for 2-4 hours, and monitor the vincine point by thin-layer chromatography to be no more than 5%. The dehydration reaction is complete. The product mainly composed of anhydrovincine was obtained. Continue in the same reactor, stirring and lowering the temperature b...
Embodiment 2
[0014] Example 2 Separation and purification of vinpocetine
[0015] The vinpocetine ethanol solution obtained in the synthesis steps is transferred to a concentrator, concentrated under reduced pressure to recover ethanol. Then add 200 L of dichloromethane and 200 L of water, transfer to an extraction tank for extraction and separation, extract and separate the dichloromethane twice, combine the organic phases, and dry with anhydrous sodium sulfate. Then transfer to a stirring concentrator to recover dichloromethane to dryness to obtain crude vinpocetine. Then add 450 L of absolute ethanol and heat to dissolve, fine filter, and the filtrate is transferred to a crystallization tank with a concentrator. After concentrating 300 L of ethanol under reduced pressure, the mixture was stirred and cooled to below 10 °C for 2 h to crystallize, filtered and dried to obtain 43.2 kg of fine vinpocetine with a weight yield of 86.4%. The purity by HPLC is 99.3%, and the content is 99.5% b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com