Gene optimization of cholesterol dehydrogenase and high-efficiency expression thereof
A technology of cholesterol and dehydrogenase, which is applied in the field of protein production by recombinant DNA technology, can solve the problems of harsh reaction conditions in the production process, high requirements for production equipment and production conditions, and low protein expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
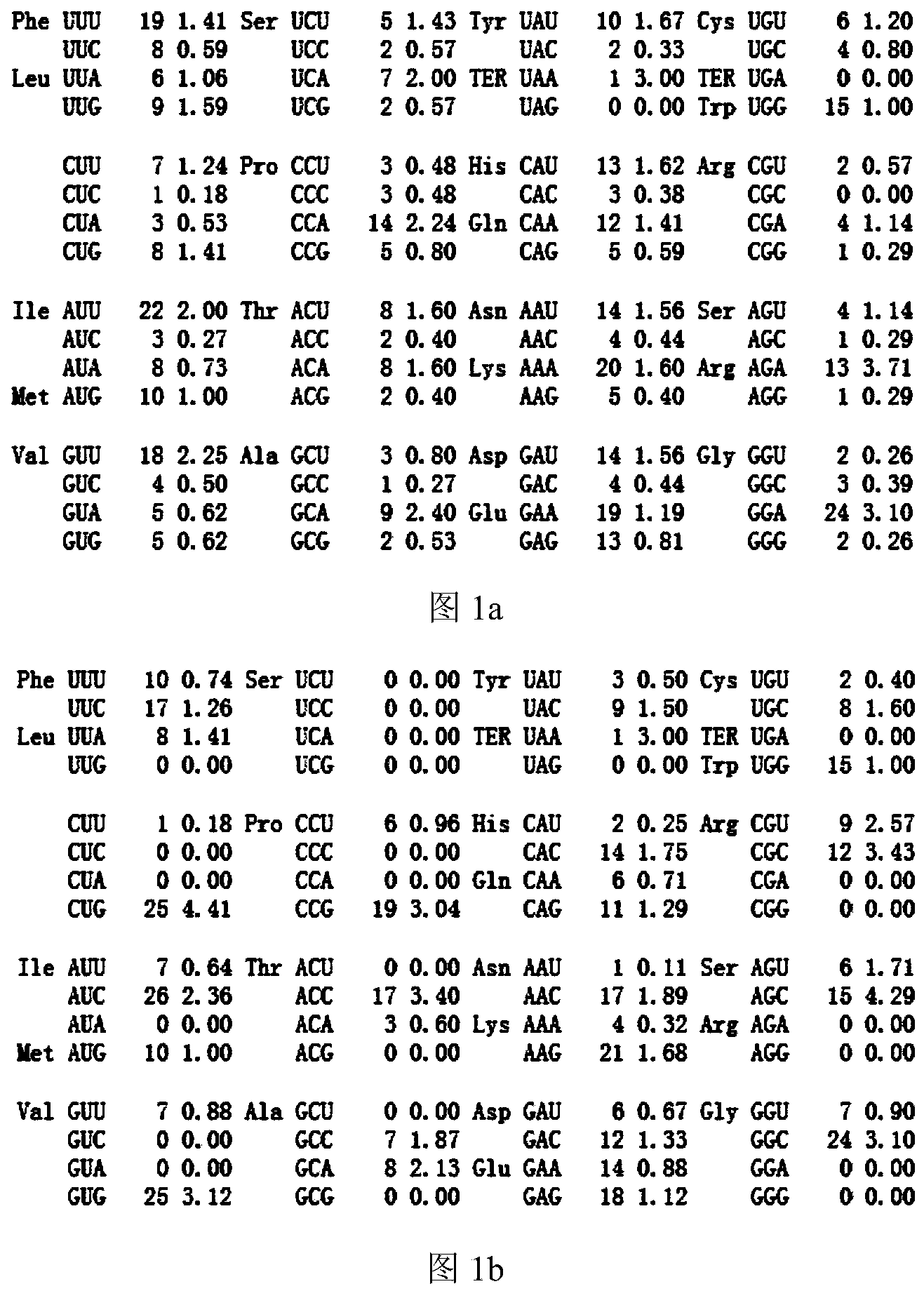
[0038] Example 1: Optimization of cholesterol 7,8-dehydrogenase gene and construction, screening and induced expression of recombinant Escherichia coli genetically engineered strains, the preparation steps are:
[0039] 1.1 Optimization design of cholesterol 7,8-dehydrogenase gene daf-36
[0040] (1) The sequence (SEQ ID NO. 1) of the original cholesterol 7,8-dehydrogenase gene (daf-36) cloned in the present invention from the nematode (Caenorhabditis elegans), the full length of the original gene is 1287bp, encoding a total of 428 amino acids and a stop codon;
[0041] (2) Through the analysis of http: / / people.mbi.ucla.edu / sumchan / caltor.html, under the premise of not changing the amino acid sequence of the protein, the preferred codons of Escherichia coli were selected;
[0042] (3) Considering factors such as the frequency of codon usage, adjustment of GC content, deletion of unstable sequences, stability of mRNA secondary structure, etc., according to the preferred codons...
Embodiment 2
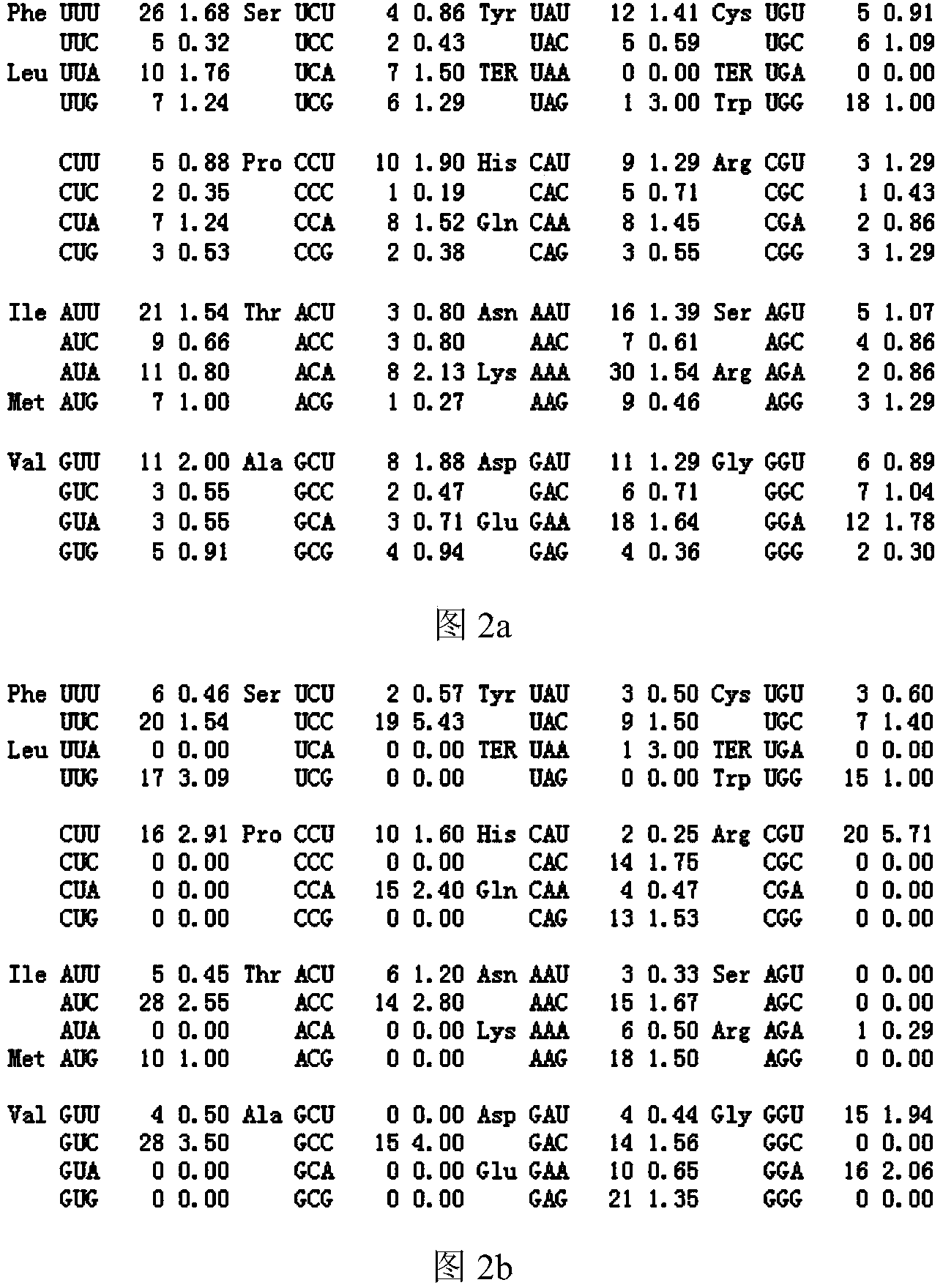
[0051] Example 2: Optimization of cholesterol 7,8-dehydrogenase gene and construction and screening of recombinant Pichia pastoris genetically engineered strains, the preparation steps are:
[0052] 2.1 Optimization design of cholesterol 7,8-dehydrogenase gene daf-36
[0053] (1) The sequence of the original cholesterol 7,8-dehydrogenase gene (daf-36) cloned in the present invention from the nematode (Caenorhabditis elegans), the full length of the original gene is 1287 bp, encoding a total of 428 amino acids and a stop codon;
[0054] (2) Through the analysis of http: / / www.jcat.de / , on the premise of not changing the amino acid sequence of the protein, the preferred codons of Pichia pastoris were selected;
[0055] (3) Considering factors such as the frequency of codon usage, adjustment of GC content, deletion of unstable sequences, stability of mRNA secondary structure, etc., according to the preferred codons of Pichia pastoris, the method of systematic codon optimization wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com