Treatment of peripheral vascular disease using umbilical cord tissue-derived cells
A peripheral vascular disease and cell technology, applied in cardiovascular system diseases, muscular system diseases, neuromuscular system diseases, etc., can solve the problems of cell population heterogeneity, stem cells cannot be obtained, and have not yet been characterized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0168] Efficacy of umbilicus-derived cells in a mouse hindlimb peripheral ischemia model
[0169] Materials and methods
[0170] Culture and isolation of umbilical cord cells. Umbilicus-derived cells (UDCs) were prepared as described in US Patent Publication 2005 / 0058631 or 2005 / 0054098. Cells were cultured for 10 or 11 passages (approximately 20 to 25 population doublings) and then cryopreserved.
[0171] Ischemia model treatment group :
[0172] group
[0173] 1 PBS, negative control
[0174] 2 Expression plasmid of vascular endothelial growth factor (pVEGF), positive control
[0175] 3 cells of cell line #1, totaling 5 x 10 5 cells
[0176] 4 cells of cell line #1, totaling 1 x 10 6 cells
[0177] 5 cells of cell line #2, totaling 1 x 10 6 cells
[0178] 6 cultured cells of cell line #1, totaling 1 × 10 6 cells
[0179] Cell line 1: U120304p10
[0180] Cell line 2: U072804A p11
[0181] Sample preparation for injection. Cells were either thawed immedia...
example 2
[0195] Endothelial network formation assay
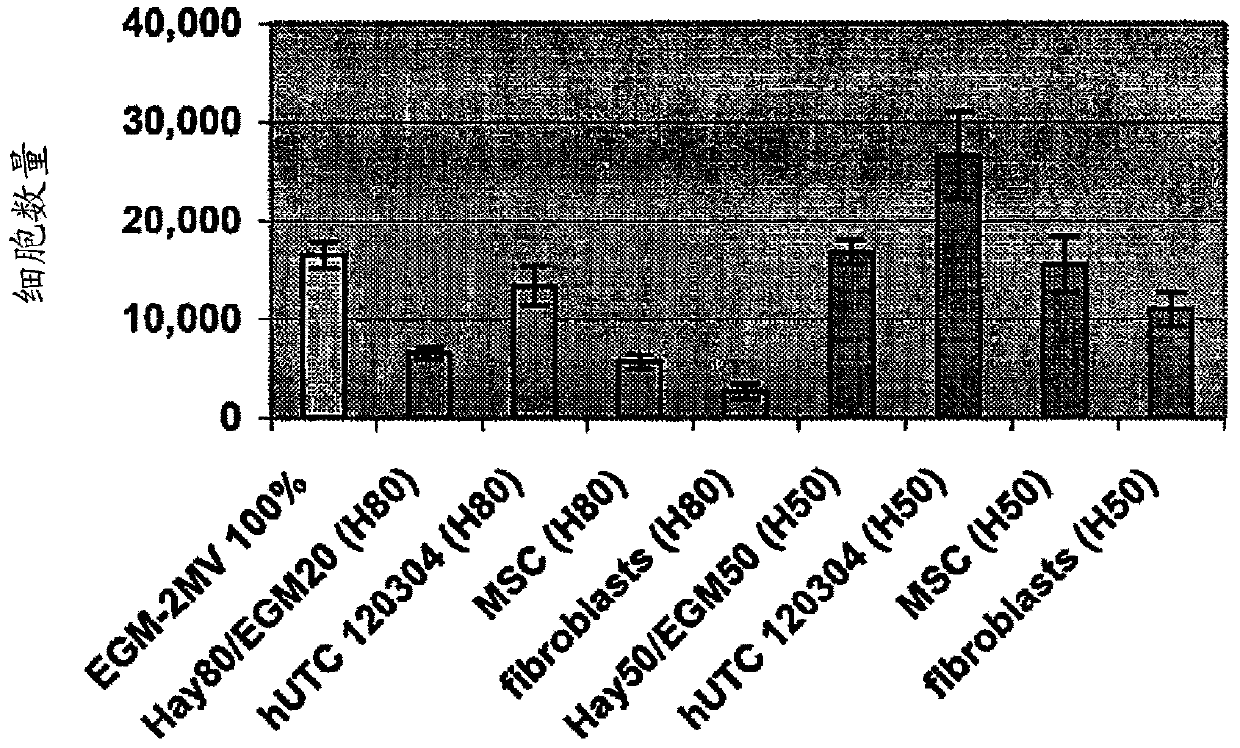
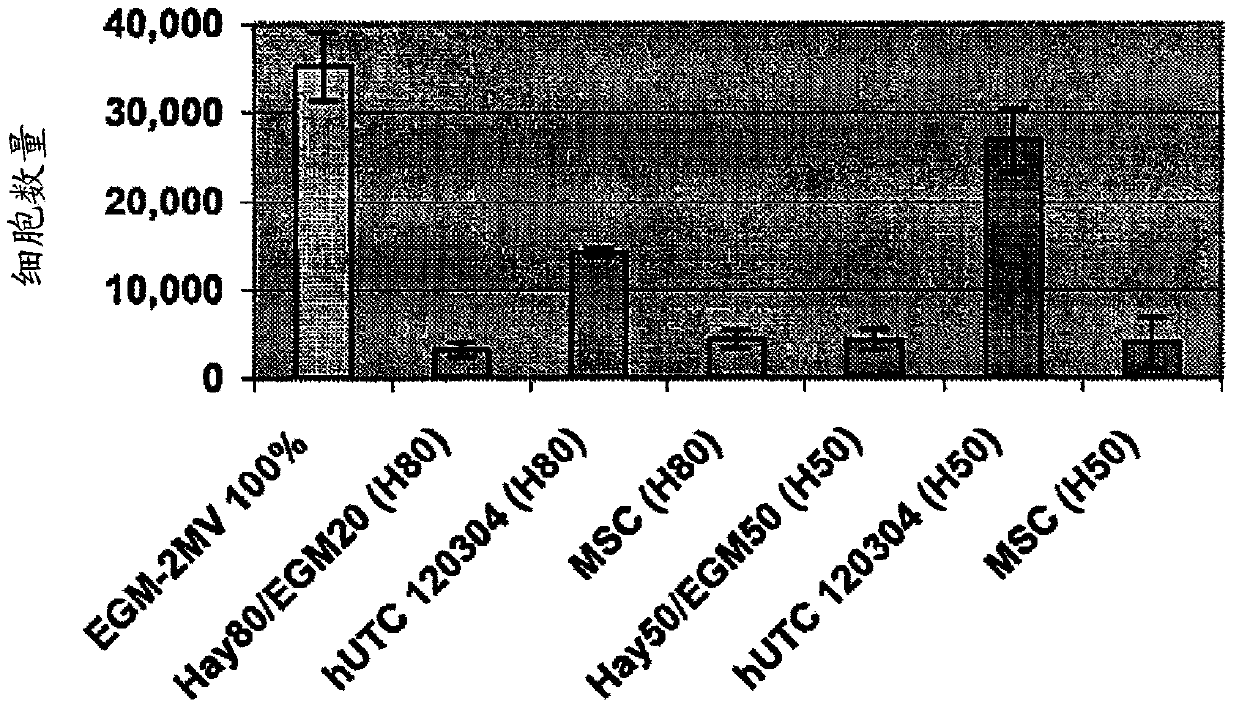
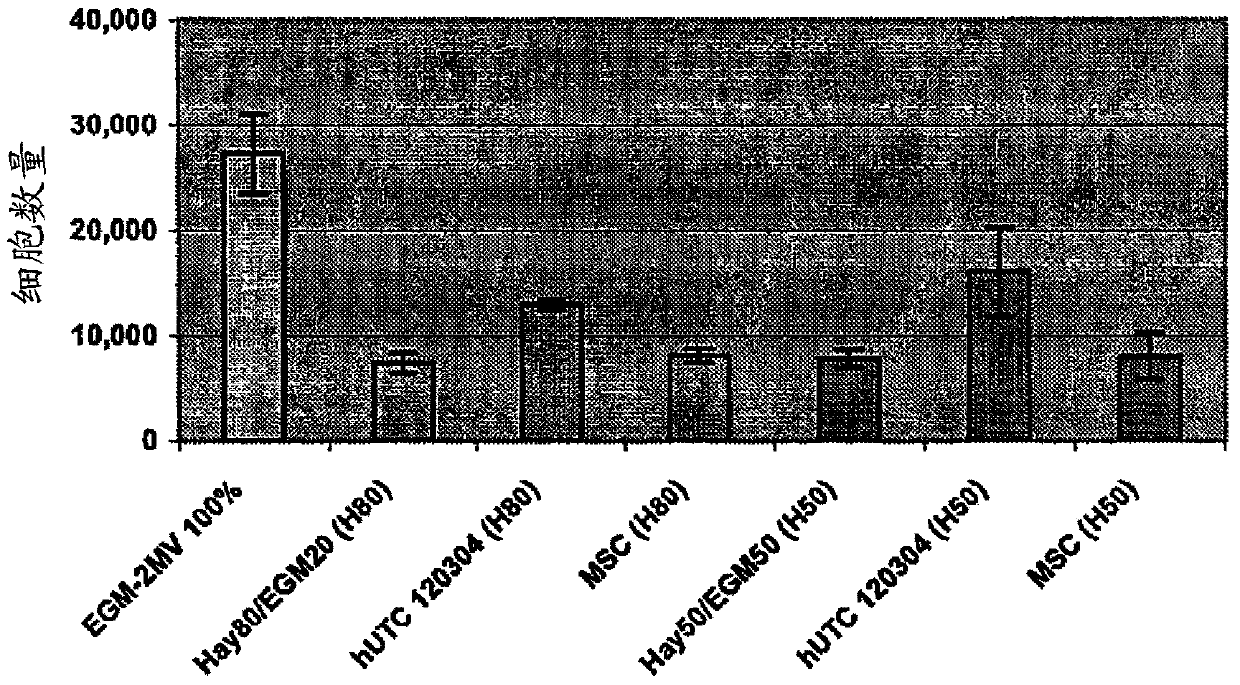
[0196] Angiogenesis, or the formation of new blood vessels, is necessary for the growth of new tissue. Induction of angiogenesis is an important therapeutic goal in many pathological conditions. For the identification of the potential angiogenic activity of umbilical cord tissue-derived cells in in vitro assays, an established method involves seeding endothelial cells onto a substrate coated with a biological cell culture (trade name MATRIGEL (BD Discovery Labware, Bedford, MA) ), and then extract the basement membrane (Nicosia and Ottinetti (1990) In Vitro Cell Dev. Biol. 26(2):119-28). Treatment of endothelial cells with angiogenic factors on MATRIGEL (BD Discovery Labware, Bedford, MA) stimulates the cells to form a network resembling capillaries. This is a commonly used in vitro assay for testing stimulators and inhibitors of angiogenesis (Ito et al. (1996) Int. J. Cancer 67(1):148-52). This experiment used a co-culture sys...
example 3
[0220] Effects of hUTC on proliferation and migration of endothelial cells in vitro
[0221] The study was conducted to determine the effect of human umbilical tissue-derived cells (hUTC) on endothelial cell proliferation and migration in vitro. These effects were examined by co-culturing hUTC and endothelial cells and by incubating human umbilical vein endothelial cell (HUVEC) cultures with hUTC lysates. The results showed that hUTC induces an increase in endothelial cell proliferation and migration. Furthermore, data suggest that these effects are mediated in part by fibroblast growth factor (FGF) and hepatocyte growth factor (HGF).
[0222] Materials and methods
[0223] cell culture
[0224] Cryopreserved human umbilical tissue-derived cells (hUTC) Lot No. 120304 were thawed at passages 8-9 and seeded on gelatin-coated bottles and cultured in Hayflick growth medium (low glucose DMEM (Gibco, cat. no. 11885-084), 15% v / v fetal bovine serum (FBS, Hyclone, catalog num...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com