2-(Alpha-hydroxyl amyl) potassium benzoate polymorphism as well as preparation method, preparation and application thereof
A technology of potassium benzoate and hydroxypentyl, applied in the field of medicine, can solve the problems of differences in physical and chemical properties, the problem of polymorphism not mentioned, affecting the application of drugs and clinical efficacy, etc., and achieve the effect of stable properties and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 —— preparation example
[0067] Embodiment 1——Preparation example: the preparation of 2-(alpha-hydroxypentyl) potassium benzoate medicinal product
[0068] Take 60 grams of crude potassium 2-(α-hydroxypentyl)benzoate, put it in absolute ethanol (1g: 2ml, w / v), heat to reflux to obtain a slightly turbid solution, and filter it while it is hot. The clarified filtrate was placed in the refrigerator after cooling, and white granular crystals were precipitated. The filtered potassium 2-(α-hydroxypentyl)benzoate was recrystallized in absolute ethanol according to the above method, repeated three times until the crystal melting point remained unchanged (mp153~155°C), and a total of 46.8g of white granular crystals were obtained ( Recrystallization yield 78%), this is 2-(α-hydroxypentyl) potassium benzoate pharmaceutical products.
Embodiment 2
[0069] Example 2——Preparation of 2-(α-hydroxypentyl) potassium benzoate crystal form I
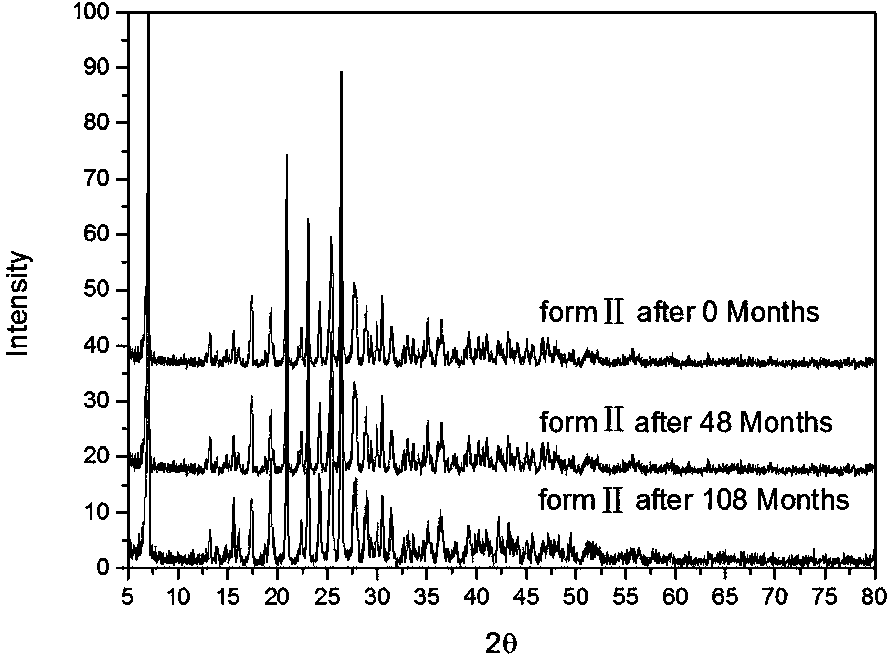
[0070] Add 10 g of potassium 2-(α-hydroxypentyl)benzoate medicinal product in Example 1 into about 19 mL of methanol, heat until dissolved, filter while hot, cool to room temperature, and precipitate crystals. After filtering and drying, it was recrystallized once with methanol according to the above method. After drying, 6.5 g of white crystals (recrystallization yield 65%) were obtained, with a melting point of 152-154°C. Carry out powder X-ray diffraction after this crystal is crushed, and the collection of illustrations is shown in the attached figure 1 , see Table 1 for the data.
Embodiment 3
[0071] Example 3——Preparation of 2-(α-hydroxypentyl) potassium benzoate crystal form I
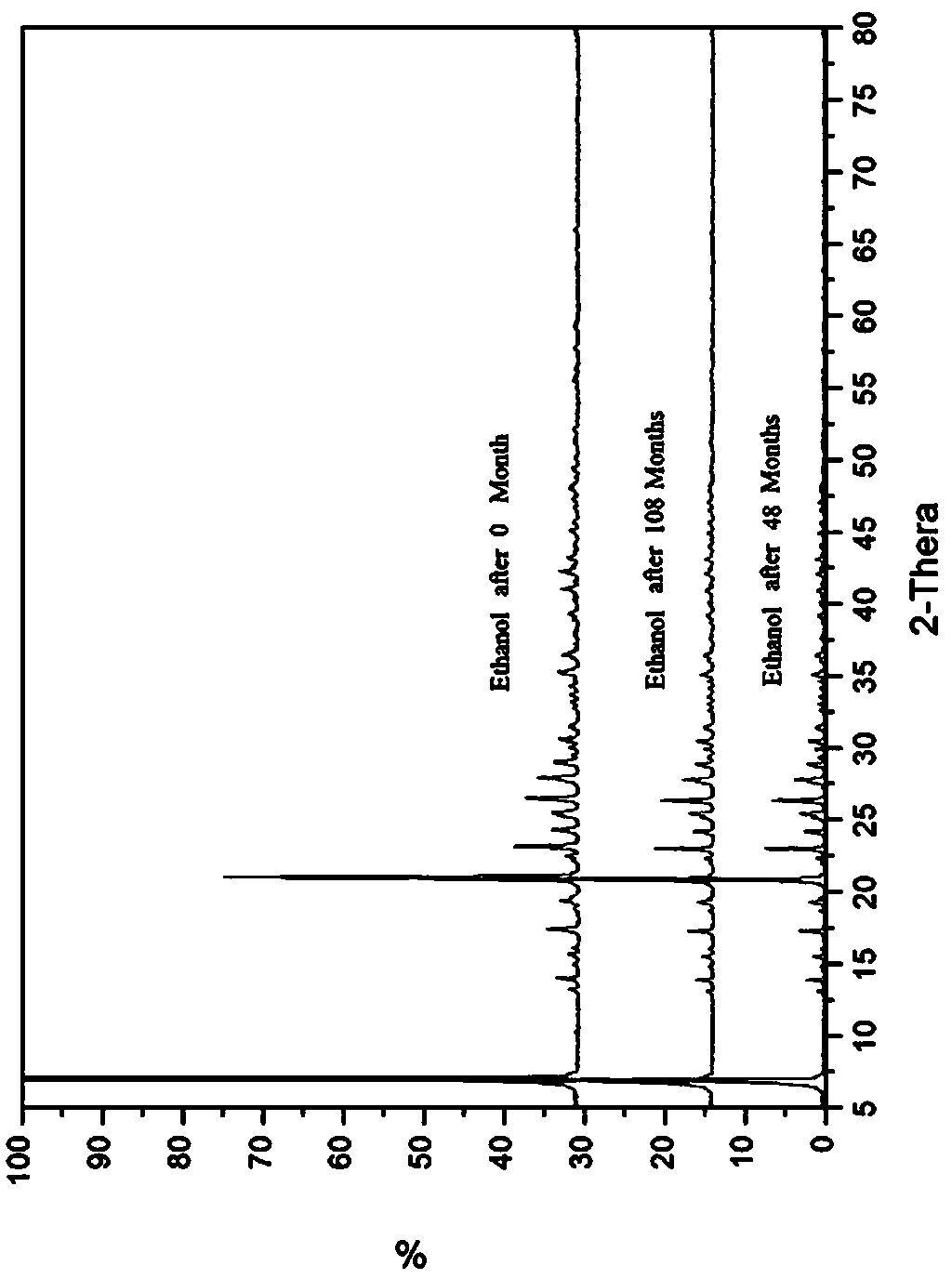
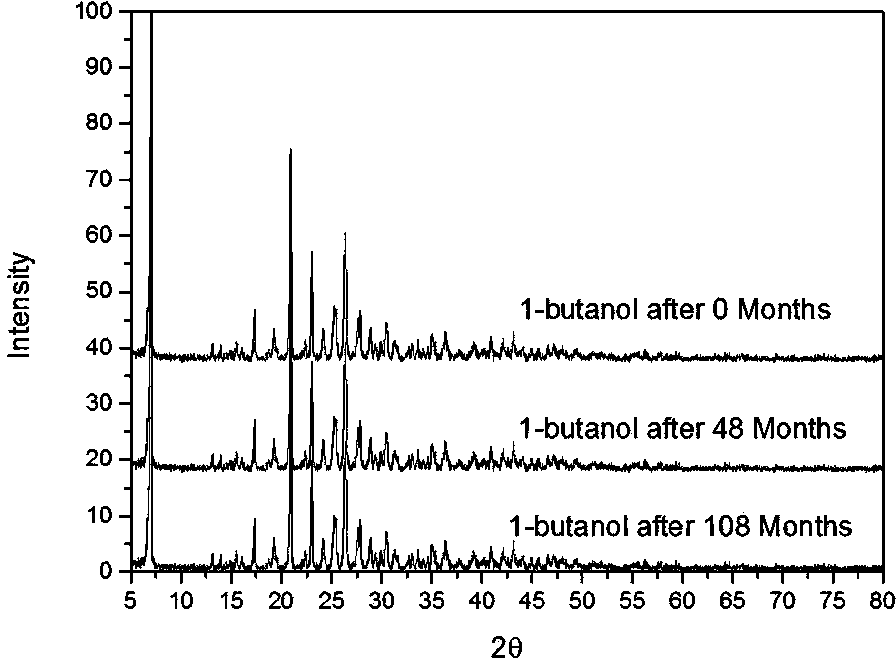
[0072] Add 10 g of potassium 2-(α-hydroxypentyl)benzoate medicinal product in Example 1 into about 20 mL of absolute ethanol, heat to dissolve, filter while hot, cool to room temperature, and precipitate crystals. After filtering and drying, recrystallize once with absolute ethanol according to the above method, and after drying, 6.7 g of white crystals (recrystallization yield 67%) were obtained, with a melting point of 153-155°C. Carry out powder X-ray diffraction after this crystal is crushed, and the collection of illustrations is shown in the attached figure 2 , see Table 1 for the data.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com