O-mercapto phenol derivatives and preparation method thereof
A technology of ortho-mercaptophenol derivatives, which is applied in the field of preparation of ortho-mercaptophenol derivatives, can solve the problems of low yield, high cost, and few researches on preparation methods of mercaptophenol derivatives, and achieve broad application prospects , complex and diverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
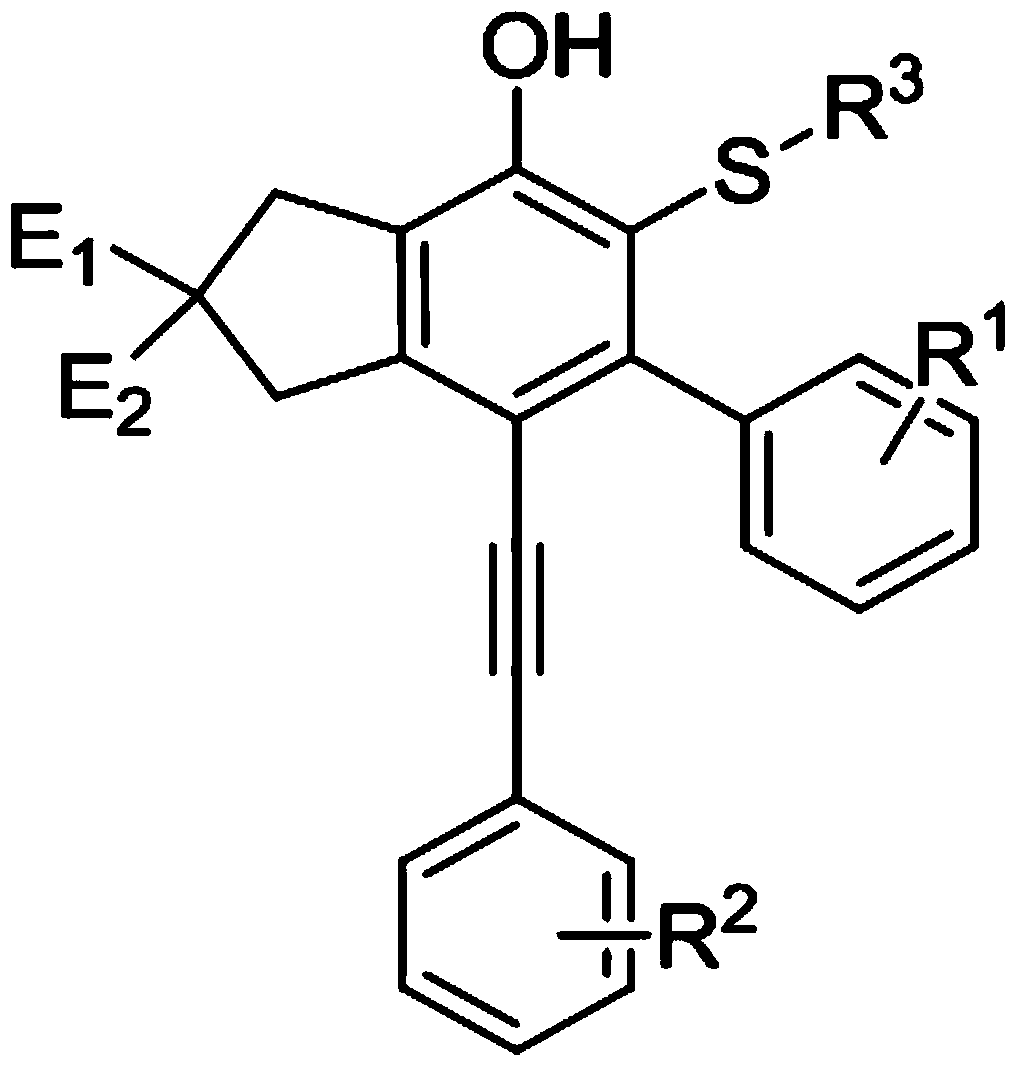
[0062] A kind of ortho mercaptophenol derivative, its structure is as follows:
[0063]
[0064] A kind of above-mentioned ortho mercaptophenol derivative, its preparation method comprises the following steps:
[0065] a. Precursor compound synthesis:
[0066] (1) Using sodium hydride (400mmol) as a catalyst, dimethyl malonate (200mmol) and propargyl bromide (440mmol) were added to anhydrous acetonitrile and stirred in an ice-water bath for 8 hours, and the reaction product was washed with water and used Extracted with ethyl acetate, spin-dried under reduced pressure, column chromatography (volume ratio ethyl acetate:petroleum ether=1:100) to obtain a white solid product, wherein the molar ratio of dimethyl malonate to propargyl bromide was 1:2.2 .
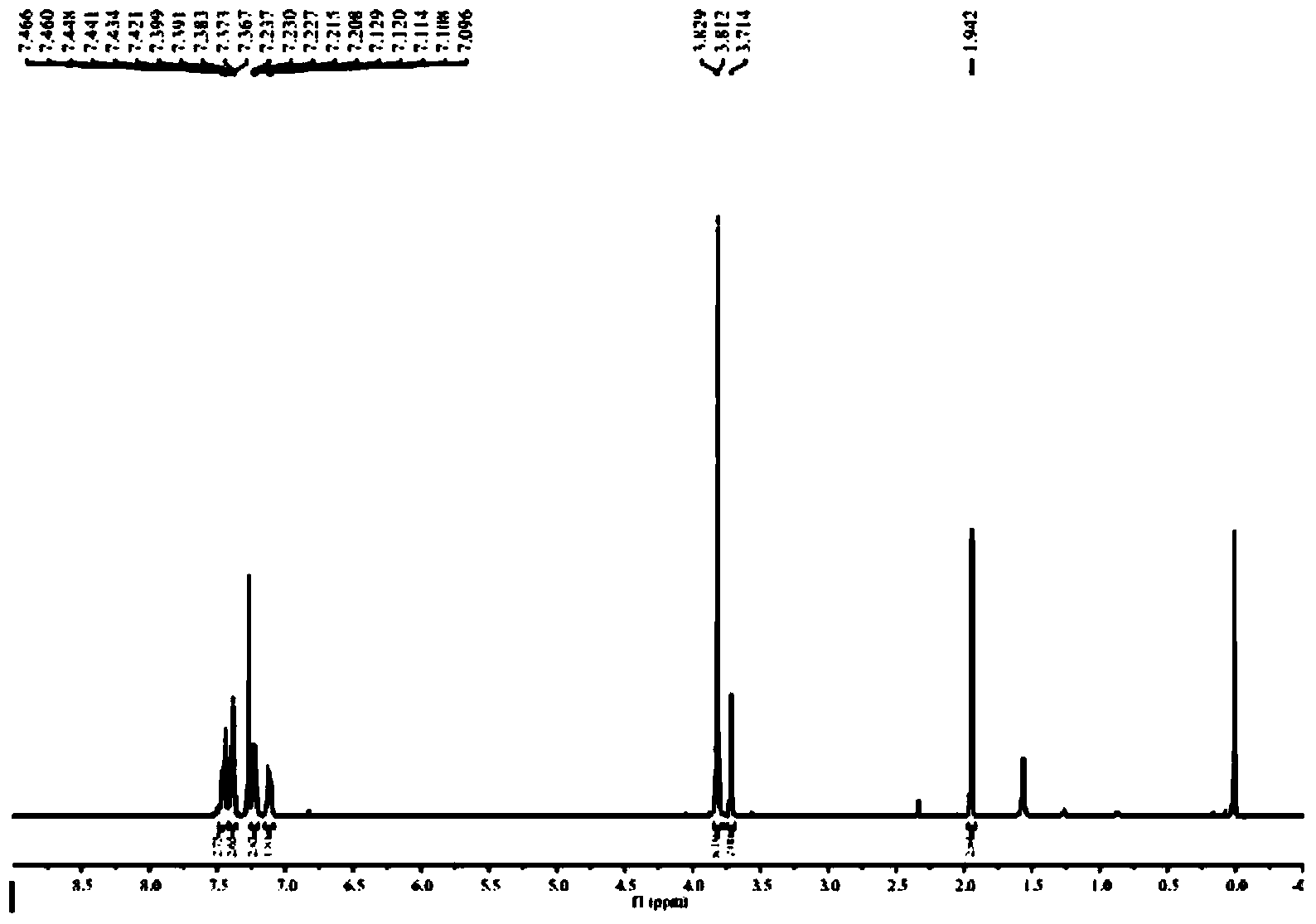
[0067] (2) The white solid product (80mmol) was mixed with phenylethynyl bromide in Pd(PPh 3 ) 2 Cl 2 : In the anhydrous and oxygen-free catalytic system of CuI=(3:1), triethylamine is used as a base, anhydrous acetonitril...
Embodiment 2
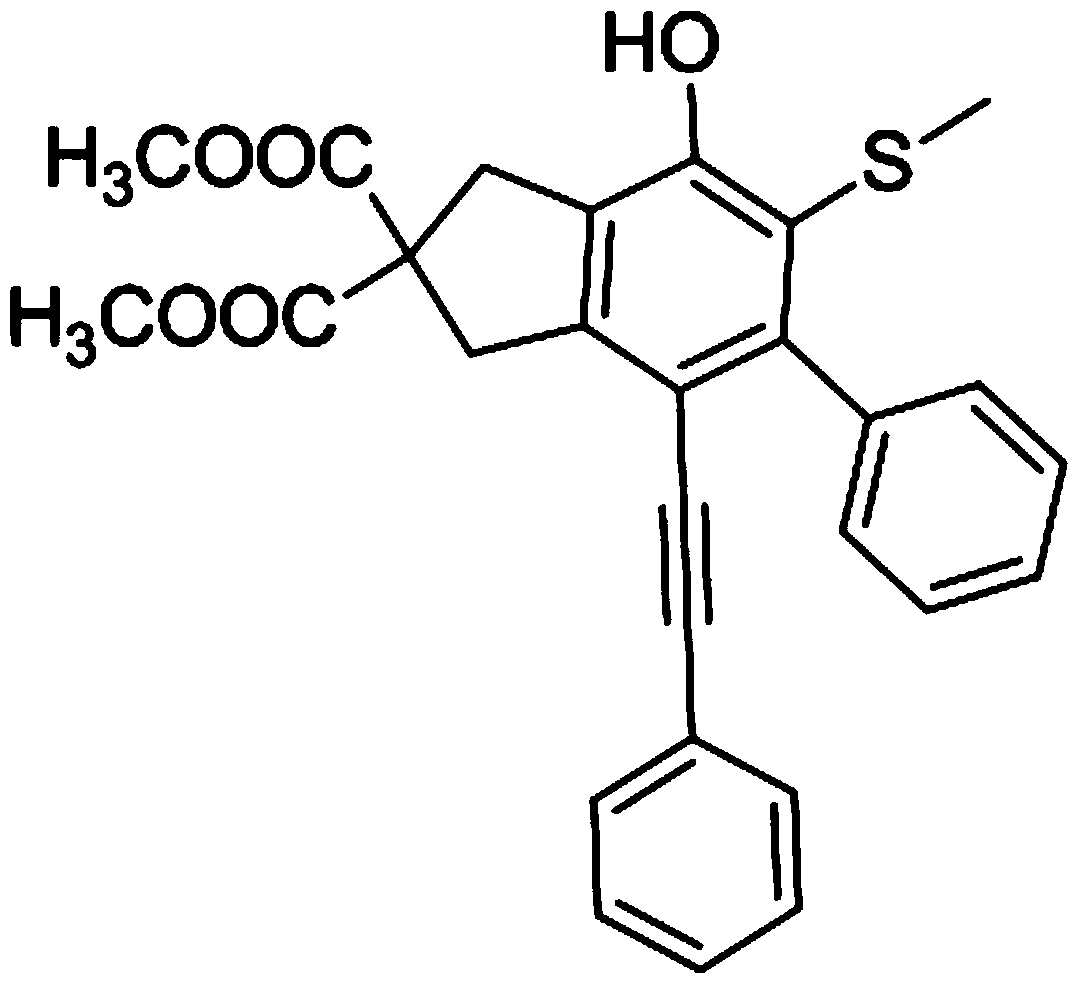
[0077] A kind of ortho mercaptophenol derivative, its structure is as follows:
[0078]
[0079] A kind of above-mentioned ortho mercaptophenol derivative, its preparation method comprises the following steps:
[0080] a. Precursor compound synthesis:
[0081] (1) Using sodium hydride (400mmol) as a catalyst, diethyl malonate (200mmol) and propargyl bromide (440mmol) were added to anhydrous acetonitrile and stirred in an ice-water bath for 8 hours, and the reaction product was washed with water and used Extracted with ethyl acetate, spin-dried under reduced pressure, column chromatography (volume ratio ethyl acetate:petroleum ether=1:100) to obtain a white solid product, wherein the molar ratio of diethyl malonate to propargyl bromide was 1:2.2 ;
[0082] (2) The white solid product (80mmol) was mixed with phenylethynyl bromide in Pd(PPh 3 ) 2 Cl 2 : In the anhydrous and oxygen-free catalytic system of CuI (3:1), with triethylamine as base, with anhydrous acetonitrile ...
Embodiment 3
[0092] A kind of ortho mercaptophenol derivative, its structure is as follows:
[0093]
[0094] A kind of above-mentioned ortho mercaptophenol derivative, its preparation method comprises the following steps:
[0095] a. Precursor compound synthesis:
[0096] (1) Using sodium hydride (400mmol) as a catalyst, dipropyl malonate (200mmol) and propargyl bromide (440mmol) were added to anhydrous acetonitrile and stirred in an ice-water bath for 8 hours, and the reaction product was washed with water and used Extracted with ethyl acetate, spin-dried under reduced pressure, column chromatography (volume ratio ethyl acetate:petroleum ether=1:100) to obtain a white solid product, wherein the molar ratio of dipropyl malonate to propargyl bromide was 1:2.2 .
[0097] (2) The white solid product (80mmol) was mixed with phenylethynyl bromide in Pd(PPh 3 ) 2 Cl 2 : In the anhydrous and oxygen-free catalytic system of CuI (3:1), with triethylamine as base, with anhydrous acetonitril...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com