Method for producing calcium metal by performing electrodeposition and refining synchronously
A metal calcium, electrowinning technology, applied in the field of molten salt electrolysis, can solve the problems of reducing current efficiency, increasing power consumption, interfering with anode operation, etc., to achieve the effect of improving purity and reducing losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
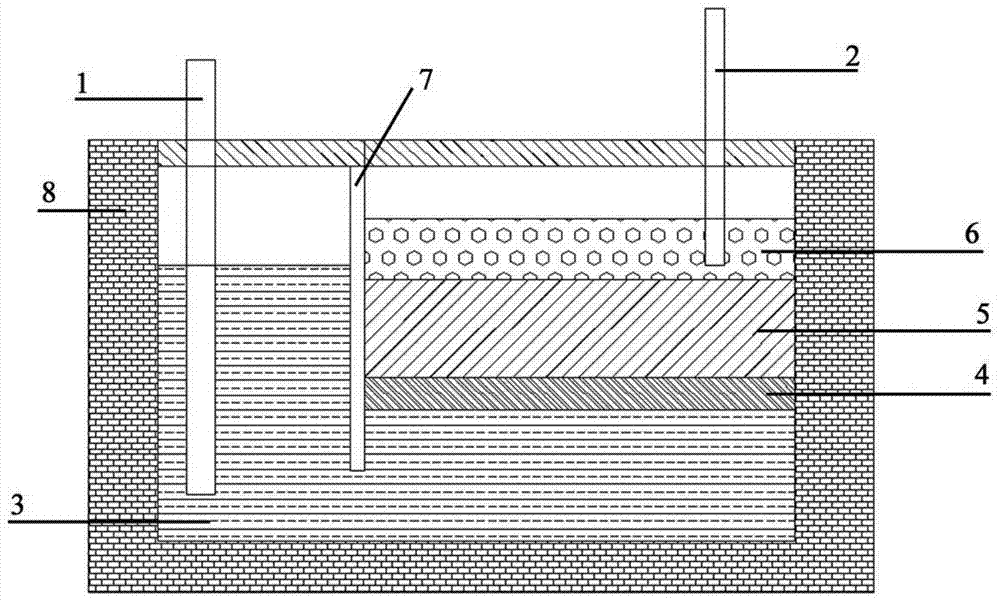
[0039] with CaCl 2 and BaCl 2 Prepare the heavy phase calcium-containing molten salt as the raw material, and the measured density at 850°C is 2.58g / cm 3. With pure CaCl 2 It is a light-phase calcium-containing molten salt with a density of 2.05g / cm at 850°C 3 . Prepare the mesophase with metal copper and metal calcium, and adjust the ratio so that the density is 2.26g / cm after it is completely melted at 850°C 3 . Under the protection of argon, the pre-melted heavy phase, intermediate phase, and light phase were respectively injected into the molten salt electrolytic cell whose temperature was stabilized at 850°C. Make it form a stable three-layer liquid structure. A graphite anode is inserted in the anode area, and an iron rod cathode is inserted in the light phase of the cathode area. Through direct current electrolysis. The cathode gradually produces metallic calcium floating on the surface of the molten salt. When metallic calcium accumulates to a certain extent,...
Embodiment 2
[0041] with CaCl 2 and BaCl 2 Prepare the heavy phase calcium-containing molten salt as the raw material, and the measured density at 850°C is 2.58g / cm 3 . with CaCl 2 Prepare light-phase calcium-containing molten salt with LiCl as raw material, and the measured density is 1.87g / cm at 850°C 3 . Prepare the mesophase with metal copper and metal calcium, and adjust the ratio so that the density after melting completely at 850°C is 2.20g / cm 3 . Under the protection of argon, the pre-melted heavy phase, intermediate phase, and light phase were respectively injected into the molten salt electrolytic cell whose temperature was stabilized at 850°C. Make it form a stable three-layer liquid structure. A graphite anode is inserted in the anode area, and an iron rod cathode is inserted in the light phase of the cathode area. Through direct current electrolysis. The cathode gradually produces metallic calcium floating on the surface of the molten salt. When metallic calcium accu...
Embodiment 3
[0043] with CaCl 2 and BaCl 2 Prepare heavy-phase calcium-containing molten salt as the raw material, and the measured density is 2.43g / cm at 800°C 3 . with CaCl 2 Prepare light-phase calcium-containing molten salt with KCl as raw material, and the measured density is 1.80g / cm at 800°C 3 . Prepare the mesophase with metal aluminum and metal calcium, adjust the ratio so that the density after melting completely at 800°C is 2.20g / cm 3 . Under the protection of argon, the pre-melted heavy phase, intermediate phase, and light phase were respectively injected into the molten salt electrolytic cell whose temperature was stabilized at 800 °C. Make it form a stable three-layer liquid structure. A graphite anode is inserted in the anode area, and an iron rod cathode is inserted in the light phase of the cathode area. Through direct current electrolysis. The cathode gradually produces metallic calcium floating on the surface of the molten salt. When metallic calcium accumulate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com