Pharmaceutical application of tauroursodeoxycholic acid and acceptable salts thereof
A technology of tauroursodeoxycholic acid and inhibitor, which can be used in medical preparations containing active ingredients, drug combinations, anti-tumor drugs, etc. There are literature reports on the physiological effects of prevention and treatment of liver cancer, etc., to achieve the effects of easy acquisition, stability verification, and wide sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
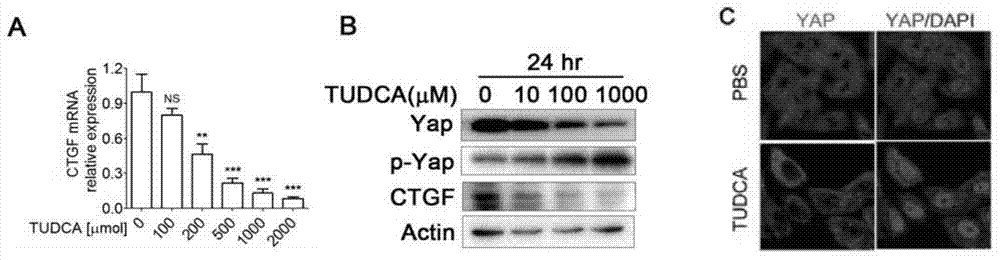
[0035] Will 1-2x10 5 HepG2 cells (purchased from Shanghai Shengbo Medical Bioengineering Technology Co., Ltd.) were plated in one well of a 6-well plate, and the HepG2 cells were treated with the control solvent DMSO (dimethyl sulfoxide) and 250 μM TUDCA on the next day, and samples were collected 24 hours later , carry out the following experiments respectively:
[0036]1) Real-time quantitative fluorescent PCR: RNA was extracted, and real-time quantitative fluorescent PCR experiments were carried out using BioRad's iQ SYBR Green Supermix kit and iCycler iQ system (BioRad, Hercules, CA, USA), using the Gapdh gene as an internal reference, and each sample was set 3 replicates, using the delta-delta method to analyze the mRNA levels of Yap and the downstream target gene CTGF (Connection tissue growth factor, connective tissue growth factor) of the transcription factor TEAD in each sample, so as to determine the effect of TUDCA on the transcriptional coactivation activity of Yap...
Embodiment 2
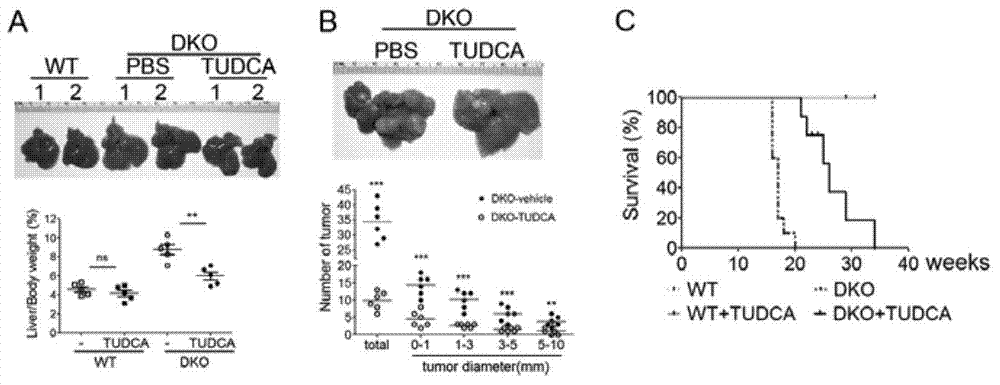
[0042] Wild-type mice (from Xiamen University Experimental Animal Center) and Mst1 / 2 gene knockout (Mst1 / 2DKO) mice (from Harvard Medical School, USA) were taken and divided into TUDCA group and control group respectively. TUDCA (250mg / kg) was injected intraperitoneally once a week, and PBS was injected intraperitoneally twice a week in the control group; mice at different treatment times were dissected in batches, the total liver volume and the number and size of tumors were observed and compared, and wild animals were collected. A part of each of Mst1 / 2 DKO mice and Mst1 / 2 DKO mice were continuously injected with TUDCA or PBS to study the effect of TUDCA treatment on the survival rate and lifespan of Mst1 / 2 DKO mice.
[0043] The results showed that in TUDCA-treated Mst1 / 2DKO mice, the liver / body weight ratio of young mice ( figure 2 A), The size and number of tumors in aged mice were significantly reduced ( figure 2 B), whereas mice treated with the control solvent had n...
Embodiment 3
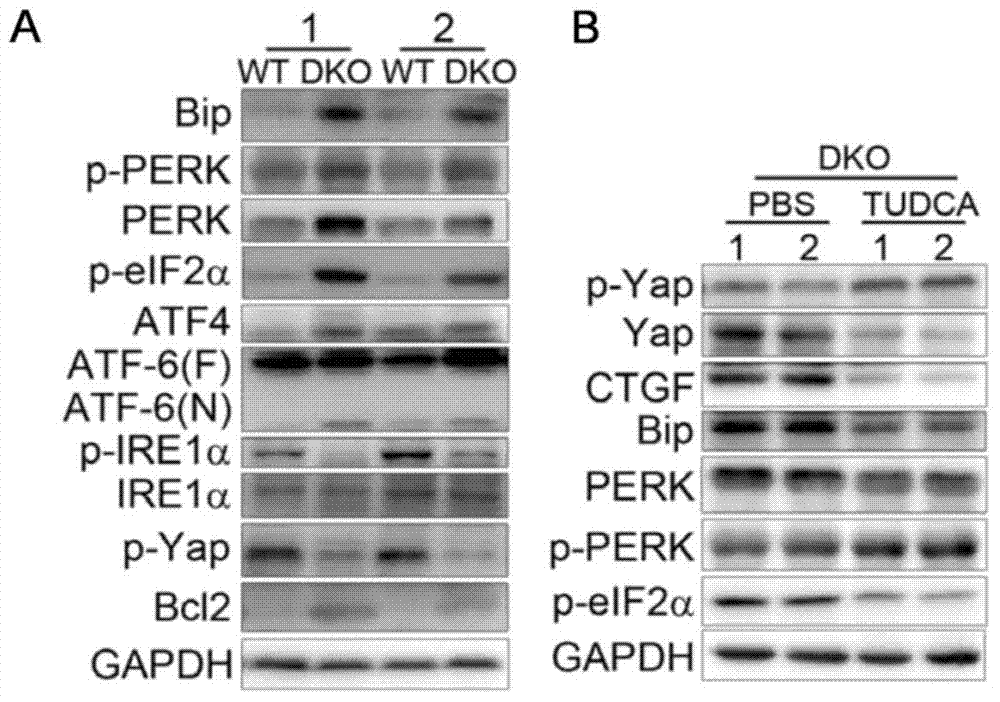
[0046] The mouse treatment method is the same as in Example 2. The expression and activity of Yap and UPR pathway elements in liver tissue samples of wild-type mice and Mst1 / 2DKO mice, and Mst1 / 2DKO mice treated with or without TUDCA (250 mg / kg) were compared by immunoblotting.
[0047] The results showed that Bip, PERK, p-PERK, p-eIF2α, and spliced ATF6 were significantly up-regulated in Mst1 / 2DKO mouse liver tissue samples compared with control wild-type mice ( image 3 A), indicating that two branches of the UPR pathway, PERK-eIF2 and ATF6-XBP1, are activated. When TUDCA treated Mst1 / 2DKO mice, compared with untreated Mst1 / 2DKO mice, the levels of Bip, PERK and p-eIF2 decreased, while p-Yap increased and CTGF decreased ( image 3 B), illustrating that both Yap activity and activated UPR pathway were inhibited by TUDCA in Mst1 / 2DKO mice.
[0048] This example illustrates that TUDCA can effectively inhibit the activity of Yap and the activation of UPR pathway in Mst1 / 2DKO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com