Catalyst for hydrofluorination reaction of acetylene to prepare vinyl fluoride and 1,1-difluoroethane, and preparation method and application thereof
The technology of difluoroethane and catalyst is applied in the field of catalyst for acetylene hydrofluorination reaction and its preparation field, and can solve the problems of carbon deposition, short service life, complicated preparation process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
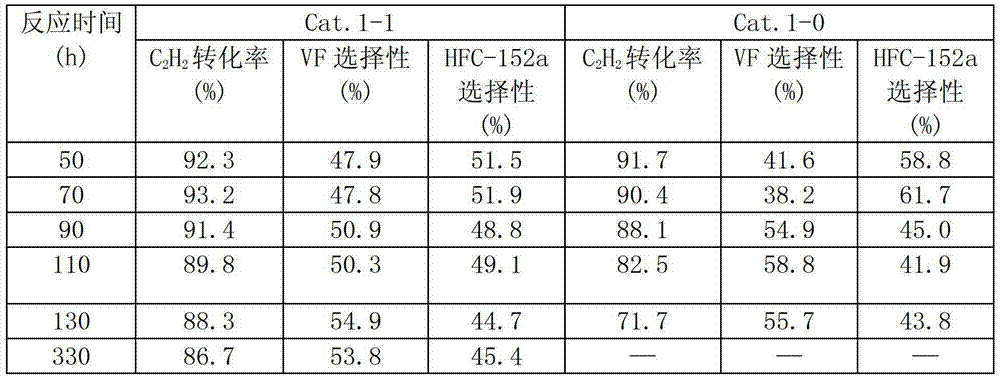
[0026] La 2 o 3 , B 2 o 3 They were respectively treated under air atmosphere at 100°C for 6 hours, and mixed at a mass ratio of 1:1 to obtain modified component A. AlF 3 , modified component A, and bentonite were mixed according to the mass percentage composition of 95%, 4%, and 1%, and were blended by ball milling, extruded by wet mixing extrusion, and dried at 105°C for 18 hours to obtain a catalyst precursor . Take 300g of the catalyst precursor and put it into a φ30*1200 carbon steel reaction tube. 2Roast at 200°C for 6 hours under protection, and then pass HF:N 2 The mixture of = 1:12 was fluorinated at 150°C for 2 hours, heated to 400°C for 2 hours, and then gradually increased the concentration of HF until pure HF was fluorinated for 4 hours to obtain the catalyst of the present invention, marked as Cat.1-1.
Embodiment 2
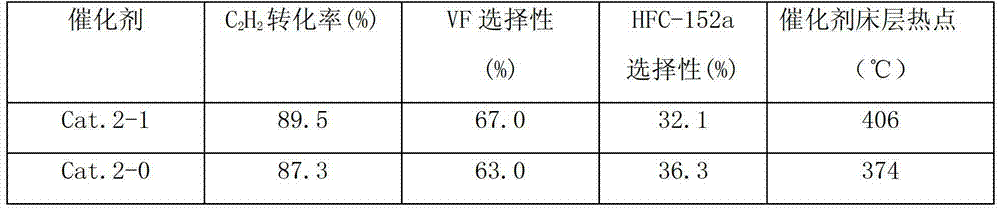
[0033] La(NO 3 ) 3 Calcined at 400° C. for 16 hours under a nitrogen atmosphere to obtain modified component A. AlF 3 , modified component A, and graphite are mixed according to the mass percentage of 85%, 10%, and 5%. After ball milling and blending, they are extruded by wet mixing extrusion and dried at 105 ° C for 24 hours to obtain a catalyst precursor. . Take 300g of the catalyst precursor and put it into a ф30*1200 carbon steel reaction tube. 2 Roast at 200°C for 16 hours under protection, and then pass HF:N 2 The mixture of =1:1 was fluorinated at 200°C for 2 hours, raised to 320°C for 2 hours, and then gradually increased the concentration of HF until pure HF was fluorinated for 4 hours to obtain the catalyst of the present invention, marked as Cat.2-1.
Embodiment 3
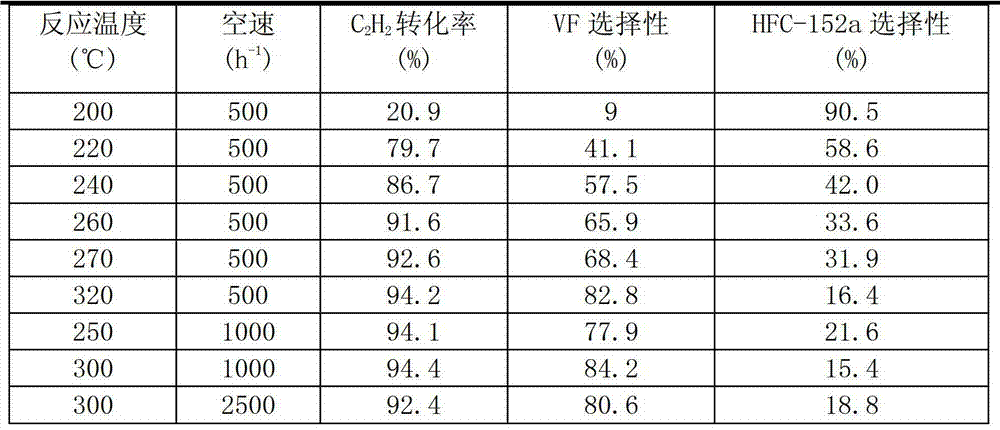
[0040] La(OH) 3 Calcined at 400° C. for 6 hours in an air atmosphere to obtain modified component A. AlF 3 , modified component A, and silicon carbide were mixed according to the mass percentage of 85%, 10%, and 5%, and were blended by ball milling, extruded by wet mixing extrusion, and dried at 120°C for 24 hours to obtain a catalyst precursor body. Get 10g of the catalyst precursor and pack it into a carbon steel reaction tube with a diameter of 14mm and a length of 600mm. 2 Roast at 200°C for 6 hours under protection, and then pass HF:N 2 The mixture of = 1:12 was fluorinated at 150°C for 2 hours, heated to 350°C for 2 hours, and then gradually increased the concentration of HF until pure HF was fluorinated for 4 hours to obtain the catalyst of the present invention, marked as Cat.3.
[0041] Catalyst Cat.3 that this embodiment makes is applied in acetylene hydrofluorination reaction, HF / C 2 h 2 The molar ratio of 1.4:1, its C 2 h 2 The reactivity and selectivity of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com