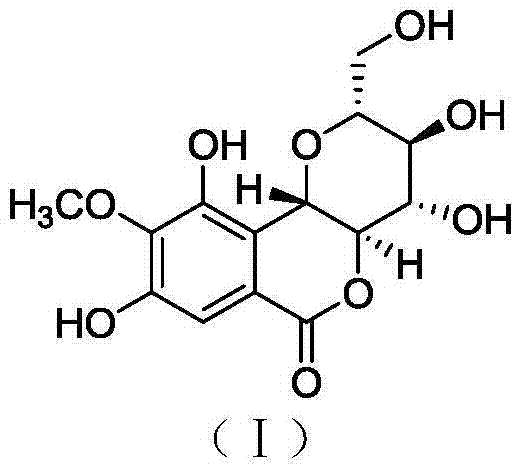
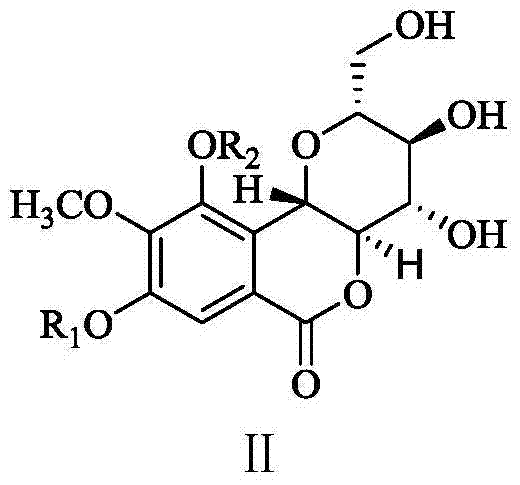
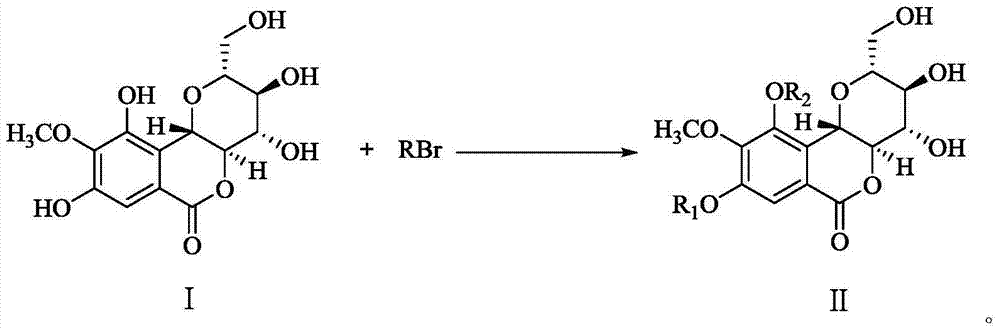
Bergenin derivatives as well as preparation method and application thereof
A technology of petrolatum and derivatives, which is applied in the field of drug synthesis, can solve problems such as unreported research on petrolatum anti-tumor activity, and achieves the effects of good operability, good reaction yield and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 18
[0023] Example 18-O-isobutyl-bergenin and 8,10-di-O-isobutyl-bergenin
[0024] Weigh petracenin (100mg, 0.30mmol), sodium bicarbonate (76mg, 0.90mmol) and catalytic amount of KI, dissolve in 5ml anhydrous DMF, add bromoisobutane (73μL, 0.67mmol) dropwise at room temperature , after the dropwise addition was completed, it was gradually heated to 80°C under the protection of nitrogen, and reacted for 12 hours; after the reaction was completed, it was cooled to room temperature, added 15ml of water and 15ml of ethyl acetate for extraction, the aqueous phase was extracted three times with ethyl acetate, and the ethyl acetate phase was combined. Wash three times with water, and wash the ethyl acetate phase with anhydrous Na 2 SO 4 After drying, the residue obtained after evaporating the organic solvent under reduced pressure was subjected to silica gel column chromatography, and the eluent was petroleum ether: ethyl acetate = 1:1. 42.8 mg of compound (2) and 65.5 mg of compound (...
Embodiment 28
[0029] Example 28-O-(2-Butyl)-Bergenin and 8,10-Di-O-(2-Butyl)-Bergenin
[0030] Weigh petracenin (100mg, 0.30mmol), sodium bicarbonate (76mg, 0.90mmol) and catalytic amount of KI, dissolve in 5ml DMF, add bromo-sec-butane (73μL, 0.67mmol) dropwise at room temperature, drop After the addition, gradually heat to 80°C under nitrogen protection, and react for 12 hours; after the reaction, cool to room temperature, add 15ml of water and 15ml of ethyl acetate for extraction, extract the water phase with ethyl acetate three times, combine the ethyl acetate phase, and wash with water Three times, the ethyl acetate phase was washed with anhydrous Na 2 SO 4 After drying, the residue obtained after evaporating the organic solvent under reduced pressure was subjected to silica gel column chromatography, and the eluent was petroleum ether: ethyl acetate = 1:1. 33.3 mg of compound (4) and 42.3 mg of compound (5) were obtained, and the yields were 28% and 31%, respectively.
[0031] 8-O-...
Embodiment 38
[0035] Example 38-O-Isopentyl-Bergenin and 8,10-Di-O-Isopentyl-Bergenin
[0036] Weigh petracenin (100mg, 0.30mmol), sodium bicarbonate (76mg, 0.90mmol) and catalytic amount of KI, dissolve in 5ml DMF, add bromoisopentane (84μL, 0.70mmol) dropwise at room temperature, drop After the addition, gradually heat to 80°C under nitrogen protection, and react for 12 hours; after the reaction, cool to room temperature, add 15ml of water and 15ml of ethyl acetate for extraction, extract the water phase with ethyl acetate three times, combine the ethyl acetate phase, and wash with water Three times, the ethyl acetate phase was washed with anhydrous Na 2 SO 4 After drying, the residue obtained after evaporating the organic solvent under reduced pressure was subjected to silica gel column chromatography, and the eluent was petroleum ether: ethyl acetate = 1:1. 40.7 mg of compound (6) and 53.7 mg of compound (7) were obtained, and the yields were 33% and 37%, respectively.
[0037] 8-O-I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com