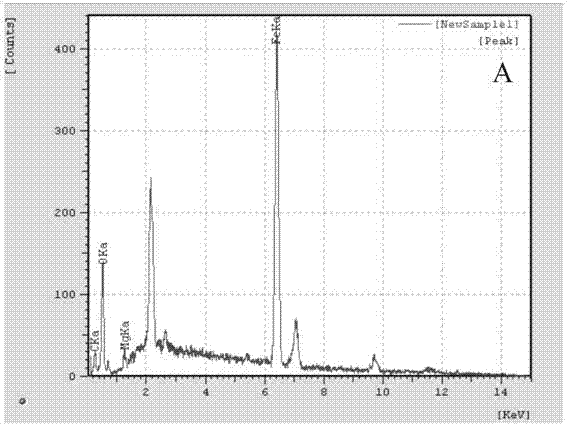
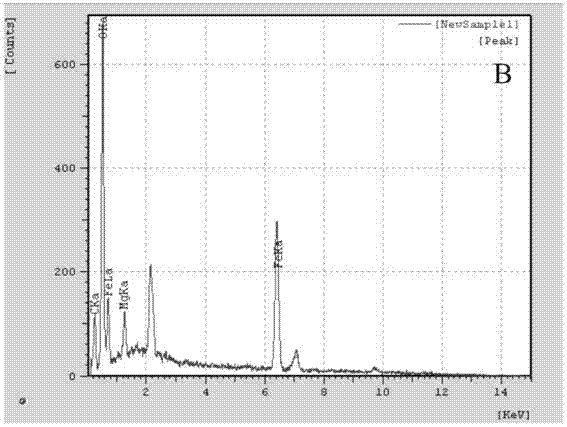
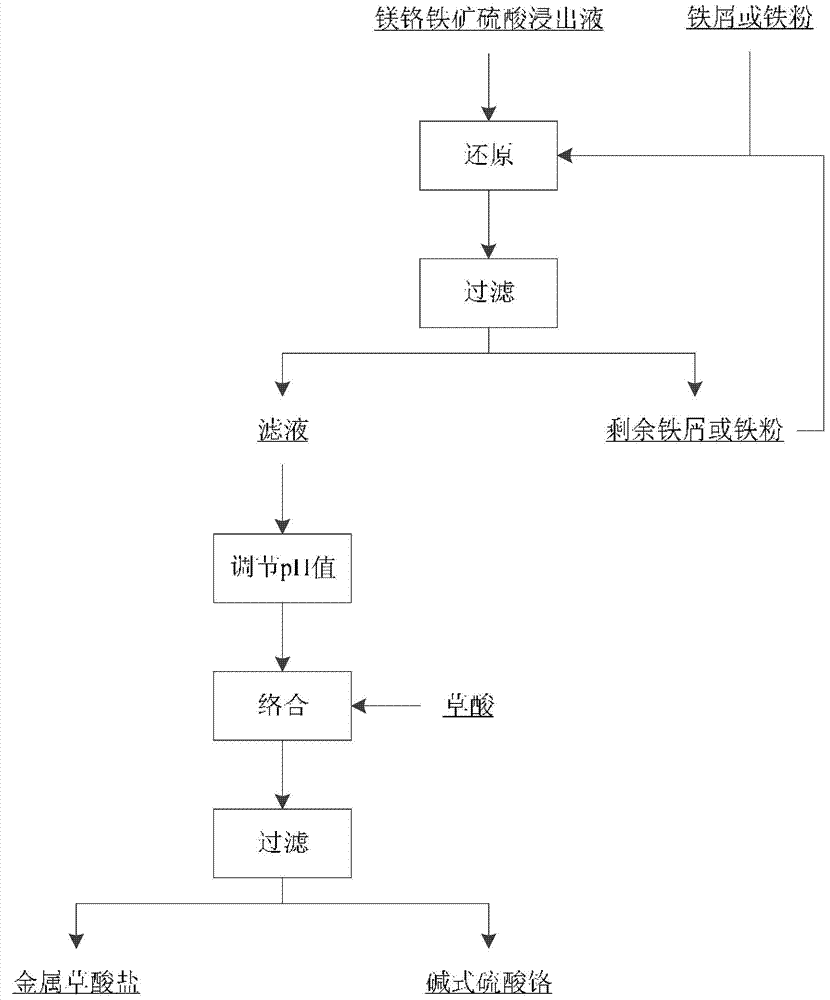
Method for preparing basic chrome sulphate by magnochromite sulphuric acid leaching solution
A magnesia chromite ore and leaching solution technology, which is applied in the direction of chromium sulfate, etc., can solve the problems such as the difficulty of removing impurities in the magnesia chromite sulfuric acid leaching solution, and achieve the effects of mild conditions, saving equipment space, and shortening the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Using 100mL magnesium chromite sulfuric acid leaching solution as raw material, the concentration of metal ions in the leaching solution is [Cr 3+ ]=34.6g / L, [Fe 3+ ]=26.6g / L, [Mg 2+ ] = 5.0 g / L.
[0032] use as figure 1 Shown magnesia chromite ore sulfuric acid leaching solution produces the schematic diagram of the technological process of basic chromium sulfate, and the present embodiment magnesia chromite ore sulfuric acid leaching solution produces the method for basic chromium sulfate, and concrete steps are as follows:
[0033] Step 1: Adjust the magnesium chromite sulfuric acid leaching solution to 70°C, place it in a constant temperature tank with a stirring device, slowly add iron filings into the container and start stirring, after 15 minutes of reaction, take 2 mL of the reaction solution every 5 minutes for use Potassium thiocyanate for Fe 3+ Detection, after 25 minutes of reaction, it was detected that there was no Fe in the reaction solution 3+ exist...
Embodiment 2
[0042] Taking 100mL of magnesium chromite sulfuric acid leaching solution as raw material, the concentration of metal ions in the solution is [Cr 3+ ]=38.1g / L, [Fe 3+ ]=24.7g / L, [Mg 2+ ]=3.4g / L.
[0043] Present embodiment magnesia chromite ore sulfuric acid leaching solution produces the method for basic chromium sulfate, and concrete steps are as follows:
[0044] Step 1: Adjust the magnesium chromite sulfuric acid leaching solution to 40°C, place it in a constant temperature tank with a stirring device, slowly add iron filings into the container and start stirring, after 35 minutes of reaction, take 2 mL of the reaction solution every 5 minutes for use Potassium thiocyanate for Fe 3+ Detection, after 10 minutes of reaction, it was detected that there was no Fe in the reaction solution 3+ exist, stop heating and stirring;
[0045] Step 2: Filter the sample to get the filtrate, at this time Fe in the filtrate 3+ Has been completely reduced to Fe by iron filings 2+ , th...
Embodiment 3
[0053] With 500mL magnesium chromite sulfuric acid leaching solution as raw material, the concentration of metal ions in the solution is [Cr 3+ ]=42.7g / L, [Fe 3+ ]=29.6g / L, [Mg 2+ ]=6.2g / L.
[0054] Present embodiment magnesia chromite ore sulfuric acid leaching solution produces the method for basic chromium sulfate, and concrete steps are as follows:
[0055] Step 1: Adjust the magnesium chromite sulfuric acid leaching solution to 90°C, place it in a constant temperature tank with a stirring device, slowly add iron powder into the container and start stirring, and after 35 minutes of reaction, take 2 mL of the reaction solution every 3 minutes and use sulfur Potassium cyanide for Fe 3+ Detection, after 15 minutes of reaction, it was detected that there was no Fe in the reaction solution 3+ exist, stop heating and stirring;
[0056] Step 2: Filter the sample to get the filtrate, at this time Fe in the filtrate 3+ Has been completely reduced to Fe by iron powder 2+ , it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com