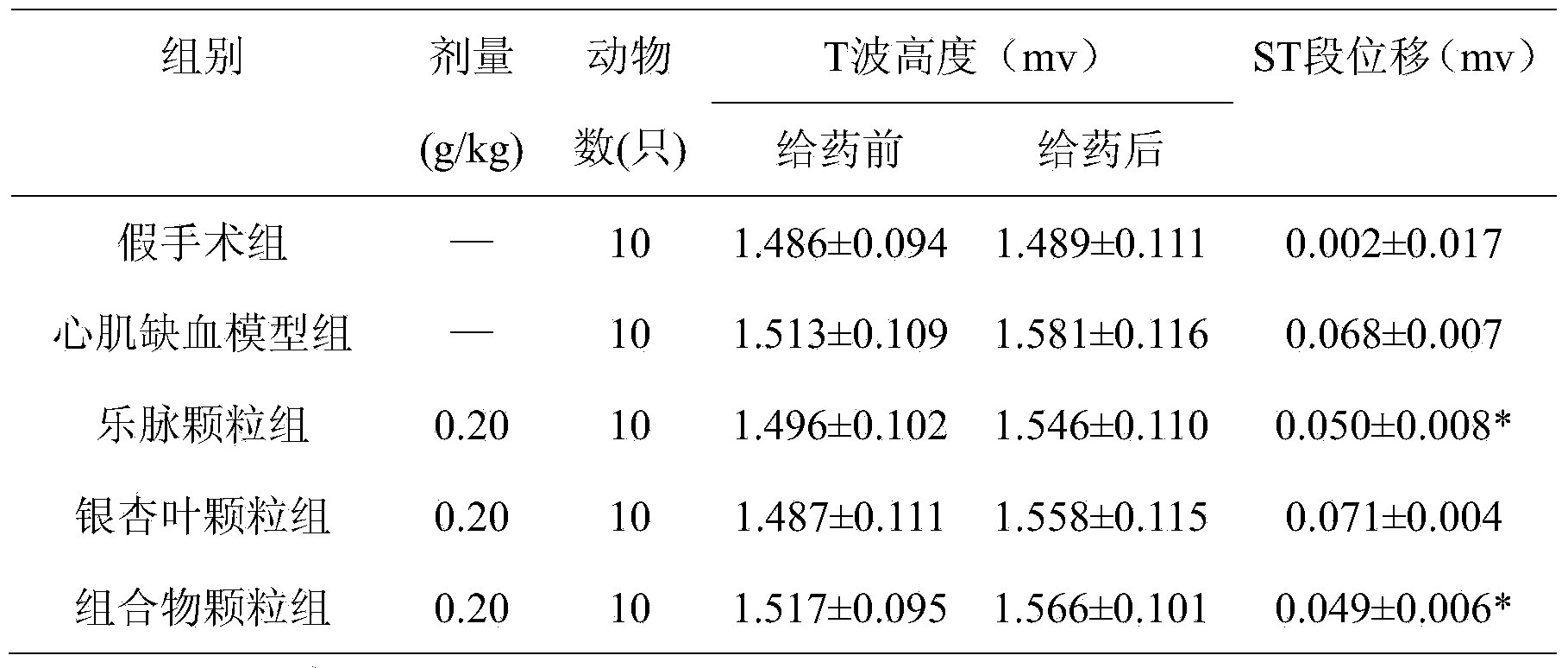
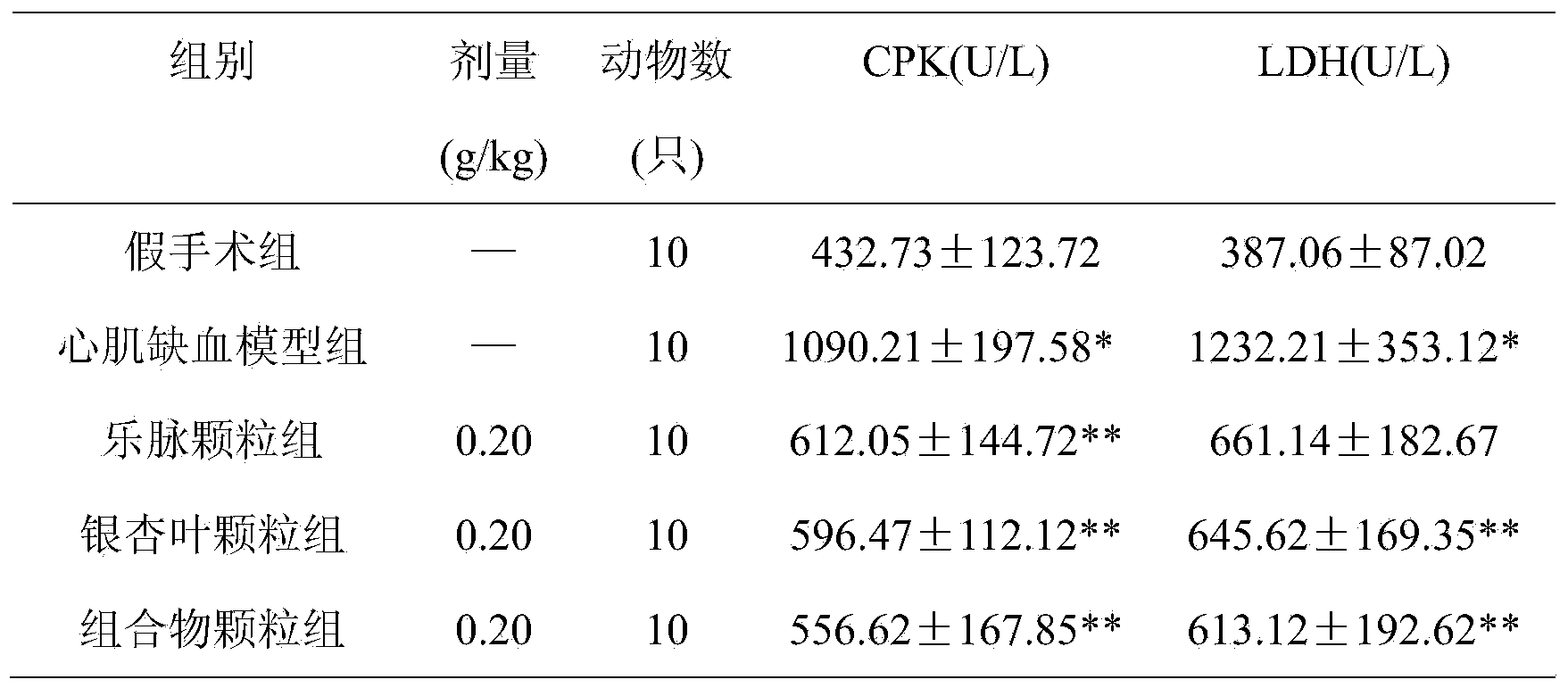
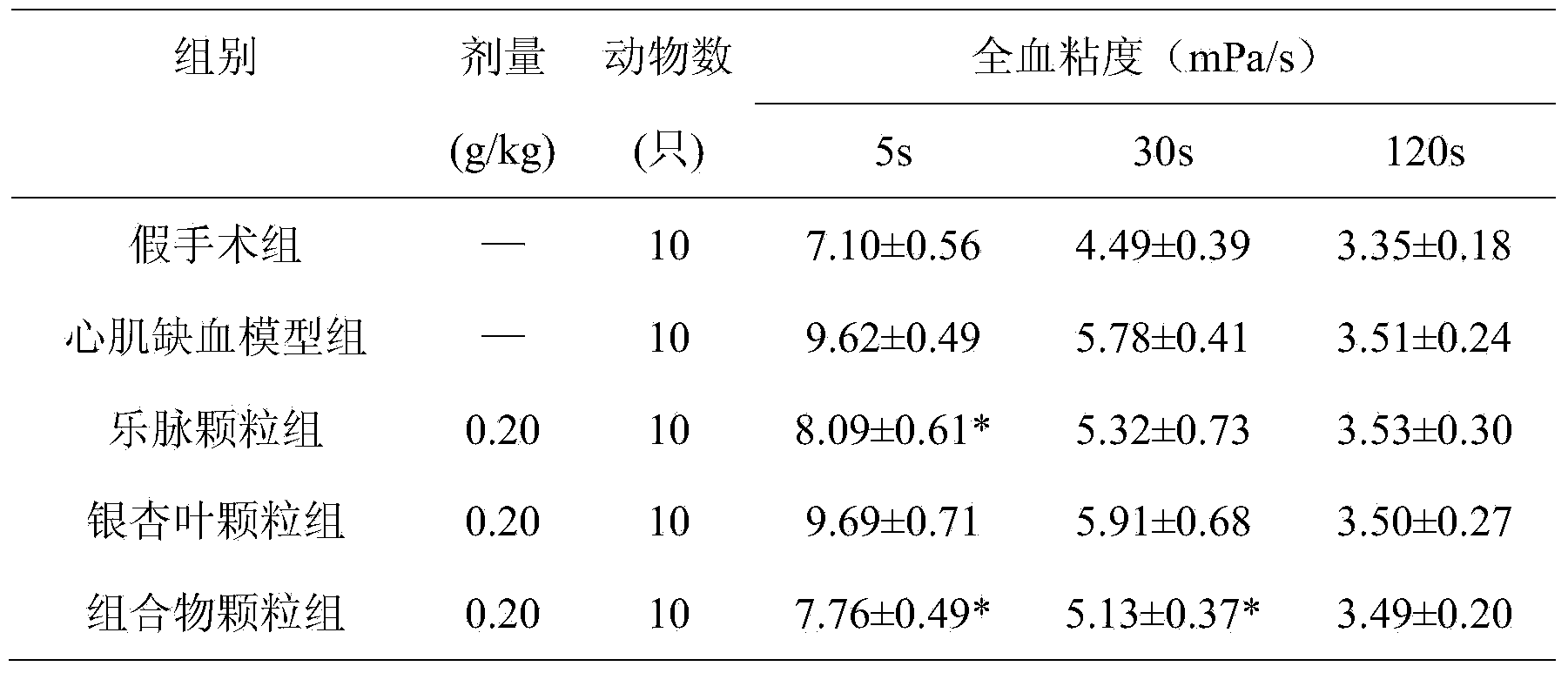
Pharmaceutical composition for treating cardiovascular and cerebrovascular diseases, as well as preparation method and application thereof
A technology for cardiovascular and cerebrovascular diseases and compositions, which is applied in cardiovascular system diseases, drug combinations, pharmaceutical formulations, etc., can solve the problems of single target, unsuitable for the prevention and treatment of cerebral infarction, and achieve the effect of enhancing blood circulation and removing blood stasis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: pharmaceutical composition pill of the present invention
[0032] Weigh 500.00g of Danshen, 260.50g of Rhizoma Chuanxiong, 260.50g of Radix Paeoniae Rubra, 260.50g of Safflower, 120.75g of Rhizoma Cyperi, 120.75g of Ginkgo biloba, 120.75g of Muxiang, and 70.40g of Hawthorn. Crush safflower, red peony and ginkgo leaves into coarse powder, sieve, and mix well. Mix the other four medicinal materials such as Salvia miltiorrhiza, add water to decoct three times, add 10 times the amount of water for the first time, and 8 times the amount for the second and third times, decoct for 1 hour each time, filter, and the filtrate is heated at 45-50 ° C. Concentrated at low temperature to a clear paste with a relative density of 1.10-1.30. Add safflower, red peony, ginkgo leaves and other coarse powders to the clear paste, mix well, dry, crush into fine powder, pour pills with appropriate amount of distilled water, dry and polish. A total of 1000 g pellets were made. ...
Embodiment 2
[0033] Embodiment 2: pharmaceutical composition oral liquid of the present invention
[0034] Weigh 450.00g of Danshen, 250.50g of Rhizoma Chuanxiong, 250.50g of Radix Paeoniae Rubra, 250.50g of Safflower, 125.75g of Rhizoma Cyperi, 125.75g of Ginkgo biloba, 125.75g of Muxiang, and 60.40g of Hawthorn. Mix the medicinal materials, add water and decoct three times, add 10 times the amount of water for the first time, and 8 times the amount for the second and third times, decoct for 1 hour each time, filter, and put the filtrate in a centrifugal thin film evaporator at a low temperature of 45-50 ℃ Concentrated to a clear paste with a relative density of 1.10-1.30. Add 300ml of 60% simple syrup and 1.5g of sodium benzoate to the obtained clear paste, adjust the pH value to 5.0, add distilled water to adjust the total amount to 1000ml, stir well, filter, pot, and sterilize with steam for 30min to obtain .
Embodiment 3
[0035]Embodiment 3: pharmaceutical composition granule of the present invention (Le Mai granule side adds Ginkgo biloba composition granule)
[0036] Weigh 490.00g of Danshen, 249.50g of Rhizoma Chuanxiong, 249.50g of Radix Paeoniae Rubra, 249.50g of Safflower, 124.75g of Rhizoma Cyperi, 124.75g of Ginkgo biloba, 124.75g of Muxiang, and 62.40g of Hawthorn. Mix the medicinal materials, add water and decoct three times, add 10 times the amount of water for the first time, and 8 times the amount for the second and third times, decoct for 1 hour each time, filter, and put the filtrate in a centrifugal thin film evaporator at a low temperature of 45-50 ℃ Concentrated to a clear paste with a relative density of 1.10-1.30. Fluidize the obtained clear paste with lactose in a batch fluidized bed, dry, add 85% ethanol soft material, pass through a 20-mesh sieve to make wet granules, dry the wet granules at 60-70°C, and dry the granules for Sieve through a 20-mesh sieve, make 1000g, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com