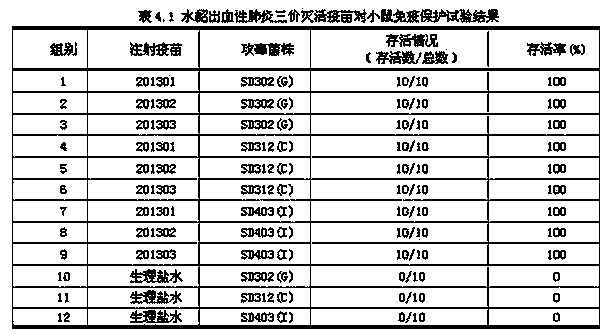
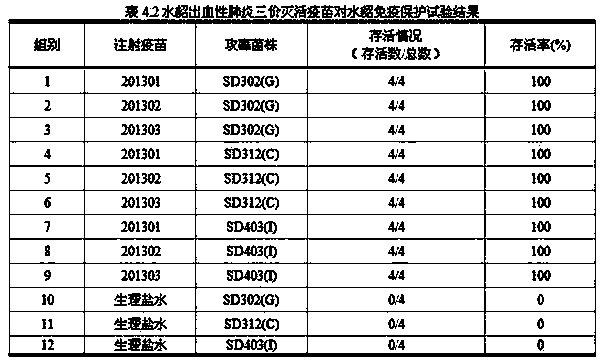
Method for preparing trivalent inactivated vaccine for preventing mink hemorrhagic pneumonia
A technique for hemorrhagic pneumonia and inactivated vaccine, which is applied in the field of preparation of trivalent inactivated vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] Example 1, preparation of inactivated vaccine
[0013] 1. Strain rejuvenation
[0014] The SD403, SD302 and SD312 strains were respectively treated with 10LD 50 Inoculate the mice. After the mice die, open the chest cavity under aseptic conditions, cut a small hole in the lung, dip it with an inoculation loop, and streak on the LB solid medium; culture at 37°C for 16 hours, pick a single colony in 5ml In PYG medium, culture at 37°C and 220 rpm for 12 hours, identify by PCR, and preserve the strains. Add 1ml of bacterial solution into 1000ml of PYG medium, incubate at 37°C and 220 rpm for 12h, count viable bacteria, and prepare seeds.
[0015] 2. Inactivated Vaccine Preparation
[0016] (1) Preparation and inspection of bacterial solution
[0017] The SD403, SD302, and SD312 secondary seed liquids that passed the inspection and viable count were inoculated at a ratio of 3% in a fermenter equipped with a PYG liquid medium, ventilated, and incubated at 37°C for 14 hour...
example 2
[0025] Example 2. Inspection of finished inactivated vaccines
[0026] 1 security check
[0027] The inspection items (sterility inspection, safety inspection, formaldehyde residue and thimerosal residue inspection, physical properties) are all carried out in accordance with the "Manufacturing and Inspection Regulations for Veterinary Biological Products" (hereinafter referred to as the "Regulations").
[0028] 1.1 Sterility test
[0029] Refer to the "Rules" for operation; after the sterility test, the three batches of vaccines 201301, 201302, and 201303 all passed the sterility test.
[0030] 1.2 Physical shape inspection
[0031] Observe the color, shape, etc. under the state of standing and shaking respectively; after shaking well, it becomes a uniform brown-yellow suspension; after standing still, the upper layer is a light yellow clear liquid, and the lower layer is a grayish-yellow precipitate;
[0032] 1.3 Inspection of formaldehyde residue and thimerosal residue
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com