Cyclopentadienyl transition metal nickel-nitrogen complex, as well as preparation method and applications thereof
A transition metal and complex technology, applied in the field of new catalytic material synthesis, achieves the effects of high yield, mild reaction conditions and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
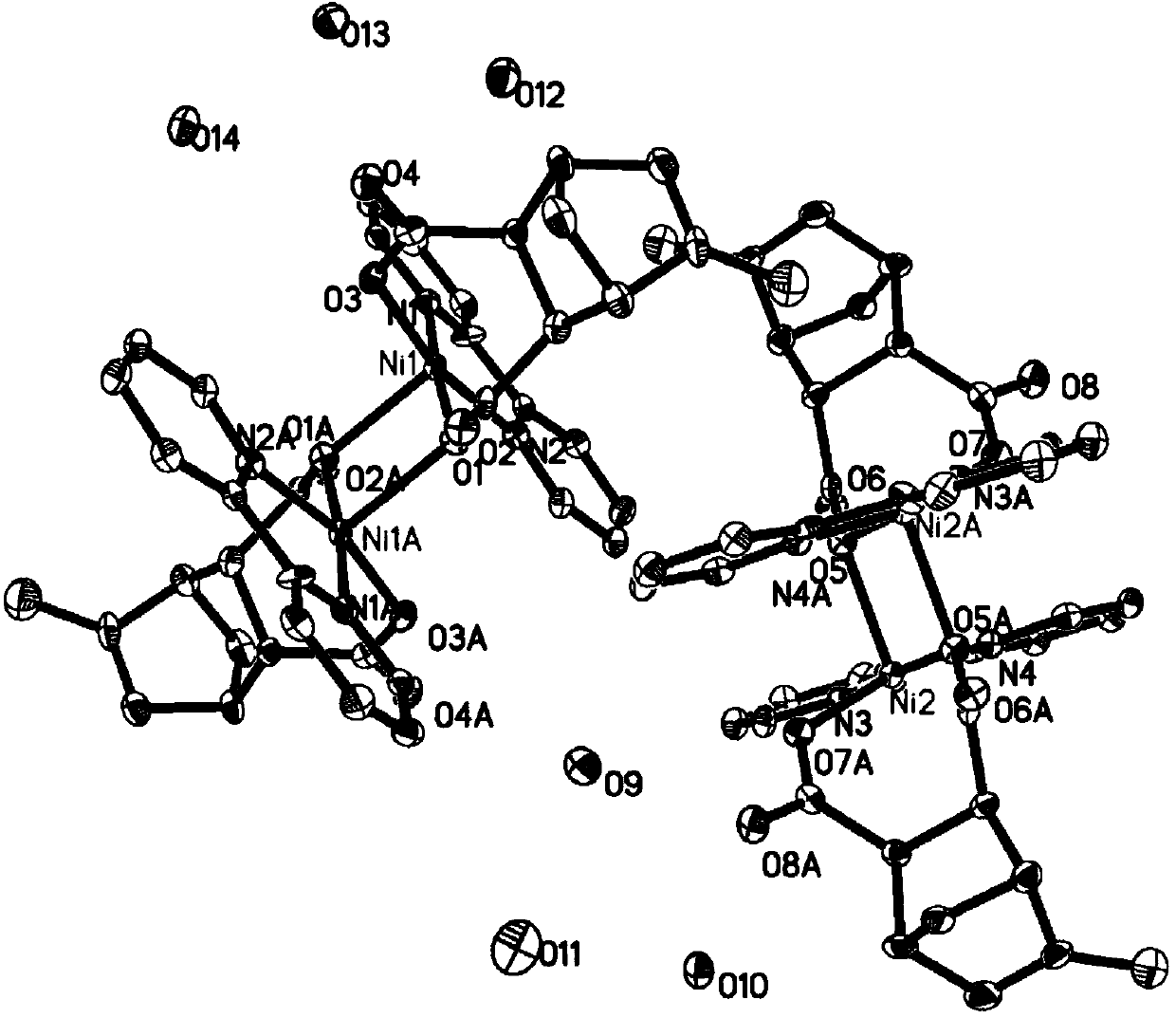
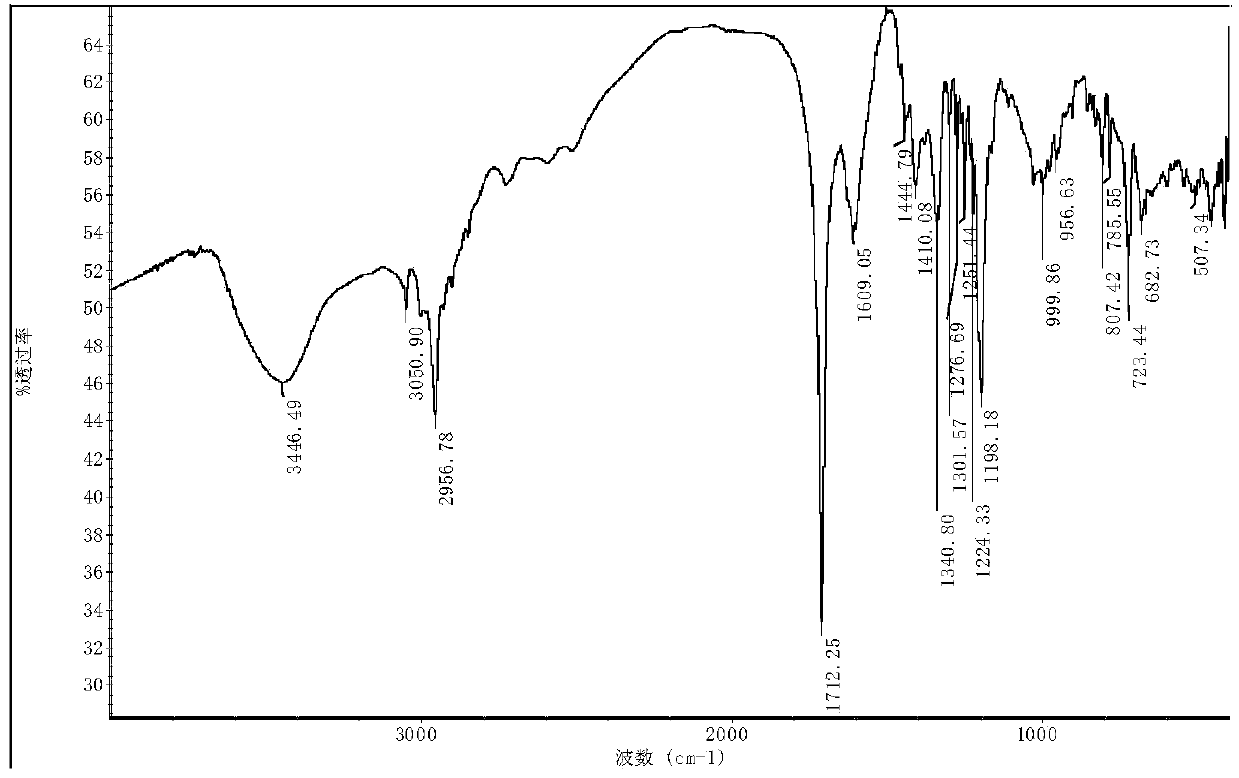
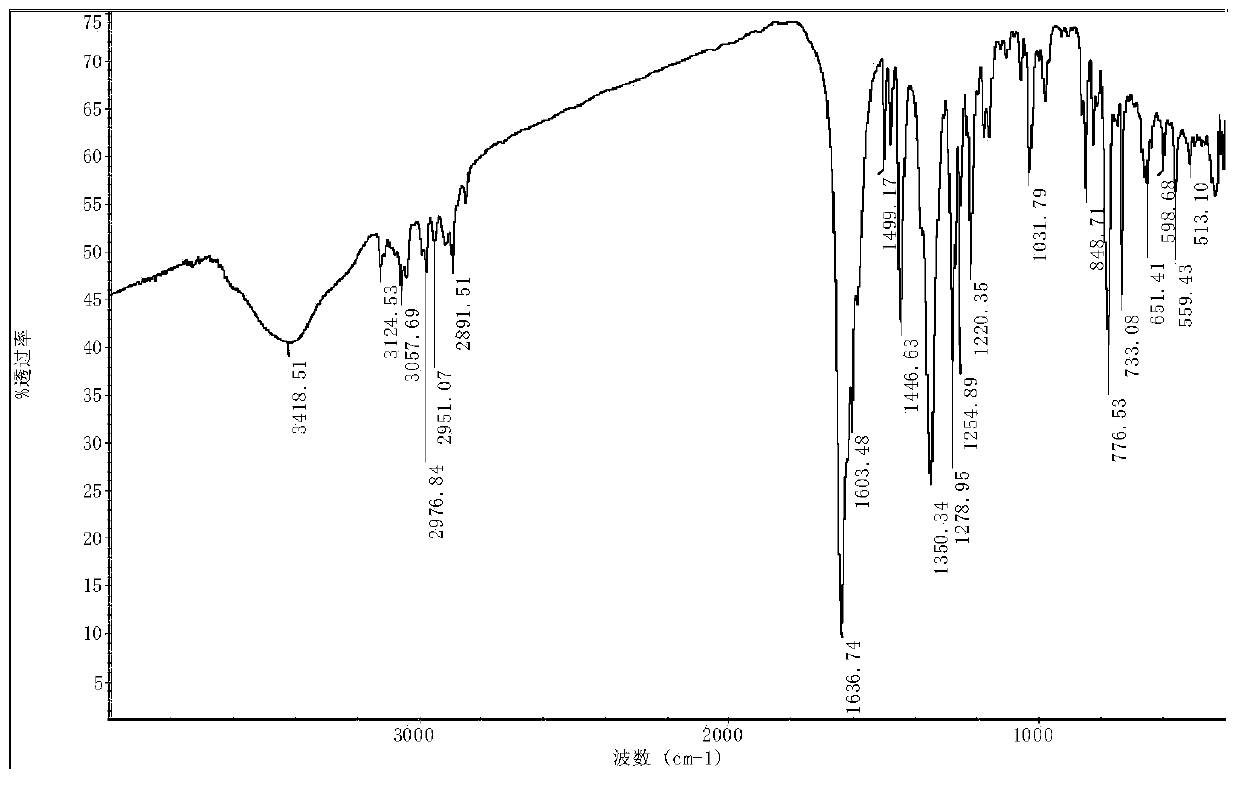
[0028] Cyclocene transition metal nickel nitrogen complex 2{Ni 2 (C 10 h 8 N 2 ) 2 [C 8 h 10 (COO) 2 ] 2} 11H 2 The synthesis of O, its reaction steps are as follows:
[0029] Add 0.36mmol (0.0645g) of methylbicyclo[2.2.1]-2-heptene-5,6-dicarboxylic acid into a 50mL round bottom flask, add 10mL of a mixed solvent of DMF and water (volume ratio of 5:2 ) dissolved, stirred to dissolve, 0.48mmol (0.1262g) nickel sulfate hexahydrate was added to the above solution, heated and stirred, the temperature was controlled at 50°C, reacted for 3h, added 0.52mmol (0.0812g) 2,2'-linked Pyridine, the temperature is controlled at 50°C, the pH value is adjusted to 6-7, the reaction is stirred for 7 hours, the reaction solution is filtered while it is hot, and after the solution is cooled, filter, and the filtrate is covered with a layer of fresh-keeping film, and placed at room temperature to evaporate naturally, after 3 weeks Obtained blue crystals. The yield is about 46.4%. Eleme...
Embodiment 2
[0031] Cyclocene transition metal nickel nitrogen complex 2{Ni 2 (C 10 h 8 N 2 ) 2 [C 8 h 10 (COO) 2 ] 2} 11H 2 Synthesis of O:
[0032] 0.42mmol (0.0752g) of methylbicyclo[2.2.1]-2-heptene-5,6-dicarboxylic acid was added to a 50mL round bottom flask, and 12mL of a mixed solvent of DMAc, ethanol and water (volume ratio of 5 :1.5:1.5) dissolved, stirred to dissolve, 0.588mmol (0.0545g) nickel hydroxide was added to the above solution, heated and stirred, the temperature was controlled at 60°C, reacted for 2h, added 0.588mmol (0.0910g)2,2 '-Bipyridyl, the temperature is controlled at 60°C, the pH value is adjusted to 5-6, the reaction is stirred for 9 hours, the reaction solution is filtered while it is hot, and after the solution is cooled, it is filtered, and the filtrate is covered with a fresh-keeping film, and placed at room temperature to volatilize naturally, 3 After a week, blue crystals were obtained. The yield is about 47.3%.
Embodiment 3
[0034] Cyclocene transition metal nickel nitrogen complex 2{Ni 2 (C 10 h 8 N 2 ) 2 [C 8 h 10 (COO) 2 ] 2} 11H 2 Synthesis of O:
[0035] Add 0.48mmol (0.0752g) of methylbicyclo[2.2.1]-2-heptene-5,6-dicarboxylic acid into a 50mL round bottom flask, add 15mL of a mixed solvent of DMF and water (volume ratio of 7:2 ) was dissolved, stirred to dissolve, 0.94mmol (0.2232g) nickel acetate was added to the above solution, heated and stirred, the temperature was controlled at 65°C, reacted for 2h, and 0.672mmol (0.1048g) 2,2'-bipyridyl was added, Control the temperature at 65°C, adjust the pH value to 6-7, stir and react for 9 hours, filter the reaction solution while it is hot, and filter it after the solution is cooled. colored crystals. The yield is about 48.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com