Application of saquinavir mesilate in preparation of drug for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
A technology for acute respiratory distress and acute lung injury, applied in the field of application of saquigravir in the preparation of drugs for the prevention or treatment of acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis, can solve the problem of increasing CD4 count, pharmacological Unreported effects, loss of binding and hydrolysis of peptides and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Preparation of Saquigravir Mesylate Lyophilized Powder for Injection
[0013] Take 50.0g of saquigravir mesylate, add 2000ml of water for injection to dissolve it, add 8g of mannitol, stir to dissolve, and ultra-filter to obtain a pyrogen-free clear liquid, pour it into a 10ml vial, 2ml / bottle, and press to freeze The dry powder injection process was freeze-dried to make each freeze-dried powder injection containing 50.0 mg of saquigravir mesylate.
Embodiment 2
[0014] Example 2 Preparation of Saquigravir Mesylate Capsules
[0015] Weigh 200.0g saquigravir mesylate, and 100.0g carboxymethyl starch sodium, mix well and pass through a 100 mesh sieve, add an appropriate amount of 3% PVP K30 Appropriate amount of water solution to make soft material, granulate with 20-mesh sieve, dry at 60°C for 3 hours, sieve with 18-mesh sieve for granulation, add 2.0g of magnesium stearate, mix well and pack into capsules, adjust the weight of the capsules to about 300mg, and the product is ready.
experiment example 2
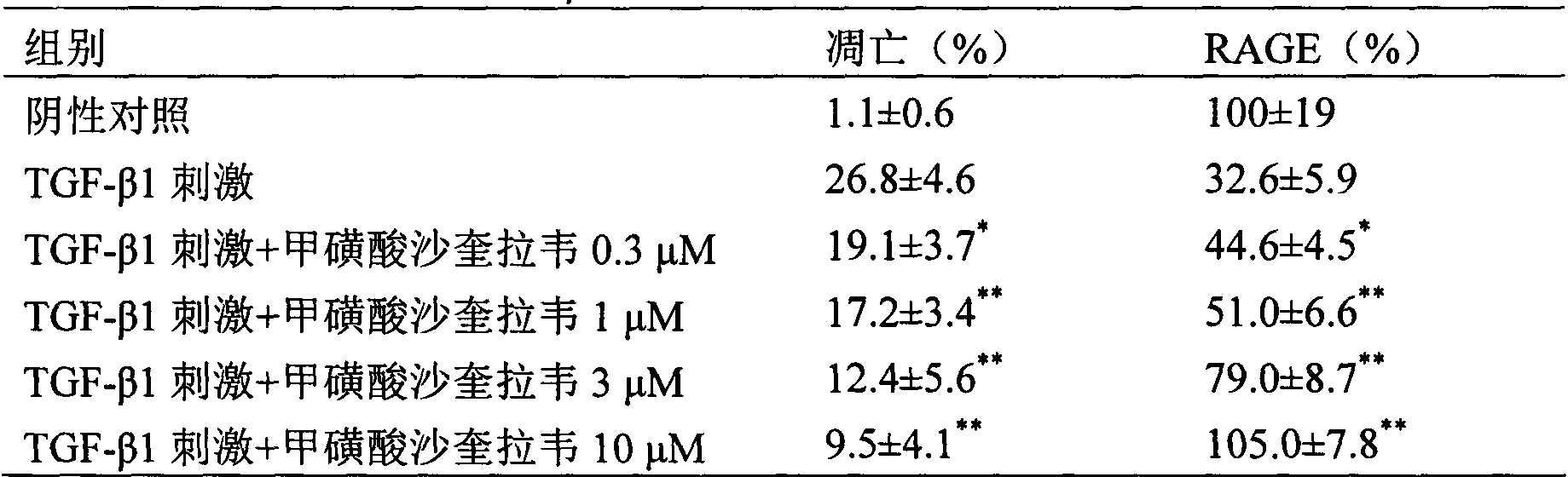
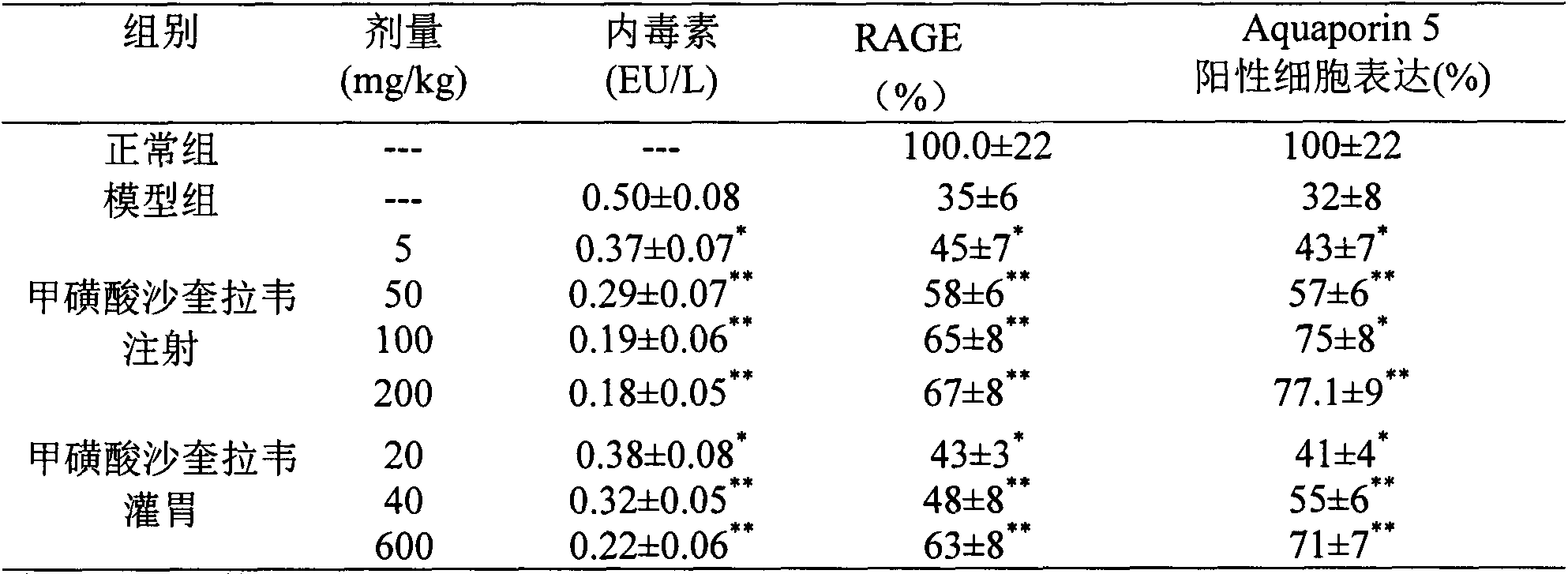
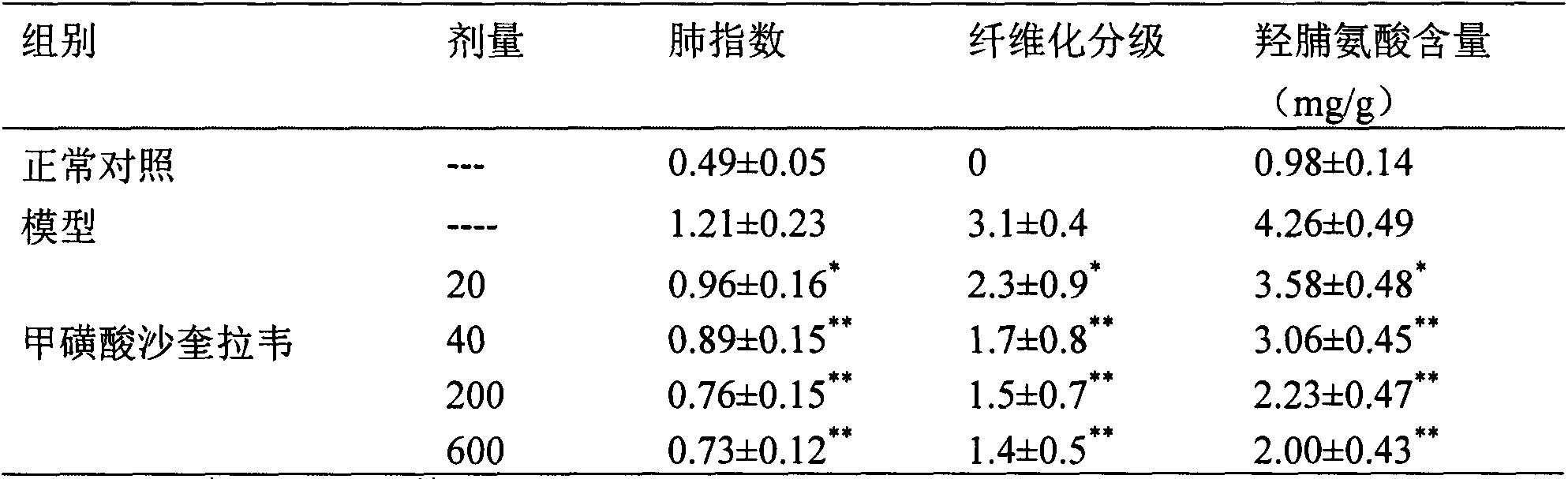
[0027] Experimental example 2 Effect of saquigravir mesylate on acute lung injury / acute respiratory distress syndrome (ALI / ARDS) model rats caused by cecal ligation and perforation
[0028] 2.1 Drugs and reagents
[0029] Saquigravir mesylate (purity 99.5%, purchased from Dalian Meilun Biotechnology Co., Ltd.)
[0030] Limulus reagent kit (Fuzhou Xinbei Biochemical Industry Co., Ltd., Fujian Province, batch number: 080430)
[0031] Anti-Aquaporin5 antibody (abcam company, ab104751)
[0032] RAGE primary antibody was purchased from Sigma.
[0033] Experimental animals: SPF grade Sprague Dawley rats, male, weighing 150g-200g, provided by the Experimental Animal Center of Shandong Luye Pharmaceutical Co., Ltd., the animal qualification certificate number is: SYXK (Lu) 20030020.
[0034] 2.2 Experimental methods and results
[0035] 2.2.1 Preparation of rats with cecal ligation and puncture (CLP)
[0036] On the day of the operation, the rats were fasted in the morning, place...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com