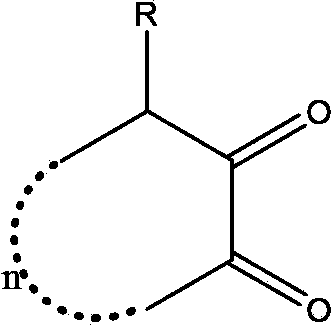
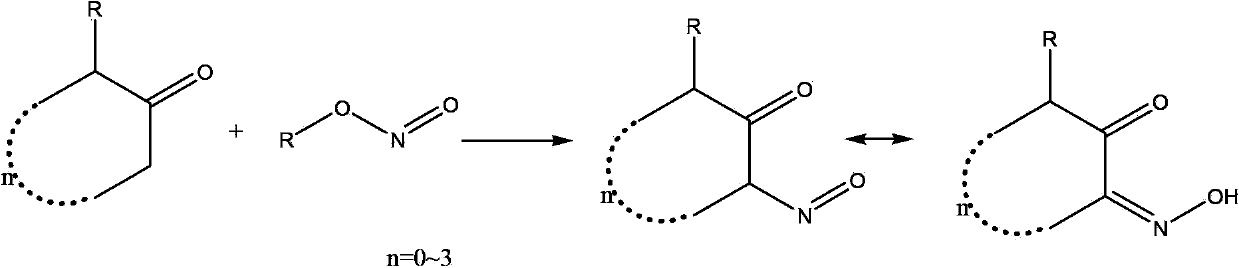
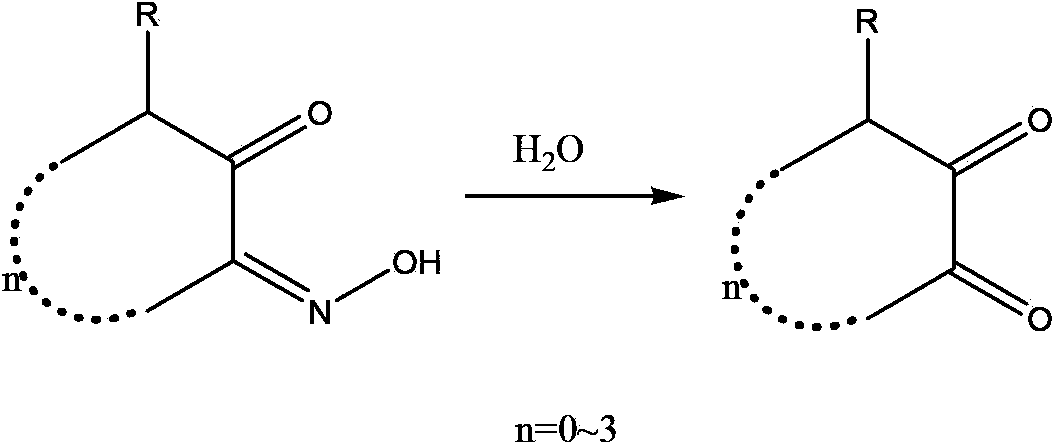
Synthetic method for o-cyclodione
A synthesis method and technology of cyclic diketones, which are applied in the fields of preparation of carbonyl compounds by hydrolysis, preparation of oximes, organic chemistry, etc., can solve the problems of cyclohexanedione without a large market supply, difficult to realize industrialization, high price, etc., and reach the production cost Low, little environmental pollution, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 100g of cyclohexanone and 5g of concentrated hydrochloric acid into a 500mL reaction flask, start stirring to make it evenly stirred, and slowly heat to 45°C. Add 130 g of isoamyl nitrite dropwise to the reaction flask within 1 hour, exothermic during the dropping process, and control the reaction within 50°C. After the dropwise addition was completed, the reaction was continued for 3 hours. After the reaction is complete, add soda ash to neutralize until the pH value is ≥ 7, and then carry out vacuum distillation. The vacuum degree is controlled at 75cmHg, the temperature is controlled below 50°C, and the distillation is carried out until no distillate is distilled. 1,2-cyclohexanedione mono 105 g of oxime crystals were precipitated, with a content of more than 98%, and a reaction yield of 81%.
[0023] (2) Add the 1,2-cyclohexanedione monoxime and 500ml of water prepared in the above step (1) into a 2000ml reaction bottle, add 70g of sodium nitrite, heat up t...
Embodiment 2
[0025] (1) Add 84g of cyclopentanone and 10g of concentrated hydrochloric acid into a 500mL reaction flask, start stirring to stir evenly, and slowly heat to 40°C. 103g of butyl nitrite was added dropwise to the reaction flask within 0.5 hours, exothermic during the dropwise addition, and the reaction was controlled within 45°C. After the dropwise addition was completed, the reaction was continued for 2 hours. After the reaction is complete, add soda ash to neutralize until the pH value is ≥ 7, and then carry out vacuum distillation. The vacuum degree is controlled at 75cmHg and the temperature is controlled below 50°C. 85g of oxime crystals were precipitated, with a content of more than 98%, and a reaction yield of 75%.
[0026] (2) Add 1,2-cyclopentanedione monoxime and 500ml of water prepared in the above step (1) into a 2000ml reaction bottle, add 75g of sodium nitrite, heat up to 40°C, and drop 800g of 50% Dilute sulfuric acid, add dropwise in about 2 hours, continue to...
Embodiment 3
[0028] (1) Add 100g of 2-methylcyclopentanone and 6g of concentrated hydrochloric acid into a 500mL reaction bottle, start stirring to make it evenly stirred, and drop 100g of isopropyl nitrite into the reaction bottle within 1 hour at room temperature. Exothermic, control the reaction within 25 °C. After the dropwise addition was completed, the reaction was continued for 2 hours. After the reaction is complete, add soda ash to neutralize until the pH value is ≥ 7, and then carry out vacuum distillation. The vacuum degree is controlled at 75cmHg, the temperature is controlled below 50°C, and steamed until no distillate is distilled. 3-Methyl-1,2- 98g of cyclopentanedione monoxime crystals were precipitated, with a content of more than 98%, and a reaction yield of 78%.
[0029] (2) Add 3-methyl-1,2-cyclopentanedione monoxime and 500ml of water prepared in the above step (1) into a 2000ml reaction bottle, add 80g of sodium nitrite, and heat up to 70°C , add 600g of 50% dilute ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com