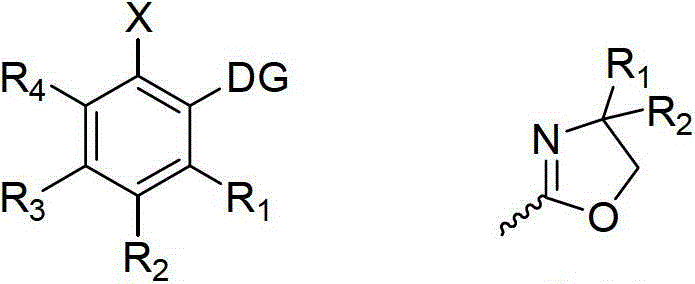A kind of halogenated aromatic ring compound and its preparation method
A technology of halogenated aromatic rings and compounds, which is applied in the preparation of carbon-based compounds, organic compounds, halogenated hydrocarbons, etc., to achieve the effects of improving synthesis efficiency, simplifying synthesis steps, and improving drug compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, preparation formula 1 compound
[0033]
[0034] Formula 1
[0035] 90mg of ethyl benzoate, 40mg of N-chlorosuccinimide, 3.4mg of Pd(OAc) 2 Add 160mg of selective fluorine reagent to a sealed tube, add 1,2-dichloroethane to dissolve; add 53uL trifluoromethanesulfonic acid under stirring, seal it, and stir at 90°C for 3h. In this reaction system, ethyl benzoate, N-chlorosuccinimide, Pd(OAc) 2 , The molar ratio of trifluoromethanesulfonic acid and selective fluorine reagent is 2:1:0.05:2.0:1.5. After the reaction was completed, 1,2-dichloroethane was added to quench the reaction, and the remaining acid and salt were washed away with saturated aqueous sodium bicarbonate solution, and extracted twice with 1,2-dichloromethane. The extracts were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. The residue was separated and purified by silica gel chromatography (n-hexane:ether (volume ratio 200:1)) to obtain ...
Embodiment 2
[0039] Embodiment 2, preparation formula 2 compound
[0040]
[0041] Formula 2
[0042] 60mg of ethyl benzoate, 36mg of N-bromosuccinimide, 2.3mg of Pd(OAc) 2 Add 106mg of selective fluorine reagent to a sealed tube, add 1,2-dichloroethane, dissolve; add 35uL trifluoromethanesulfonic acid while stirring, seal, and stir at 90°C for 4h. In this reaction system, ethyl benzoate, N-chlorosuccinimide, Pd(OAc) 2 , The molar ratio of trifluoromethanesulfonic acid and selective fluorine reagent is 2:1:0.05:2.0:1.5. After the reaction was completed, 1,2-dichloroethane was added to quench the reaction, and the remaining acid and salt were washed away with saturated aqueous sodium bicarbonate solution, and extracted twice with 1,2-dichloromethane. The extracts were combined, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. The residue was separated and purified by silica gel chromatography (n-hexane:ether (volume ratio 200:1)) to obtain 35 mg of...
Embodiment 3
[0046] Embodiment 3, preparation formula 3 compound
[0047]
[0048] Formula 3
[0049] 88mg of ethyl p-toluate, 40mg of N-chlorosuccinimide, 3.4mg of Pd(OAc) 2 Add 108mg of sodium persulfate to a sealed tube, add 1,2-dichloroethane to dissolve; add 40uL trifluoromethanesulfonic acid while stirring, seal it, and stir at 70°C for 4h. In this reaction system, ethyl p-toluate, N-chlorosuccinimide, Pd(OAc) 2 , The molar ratio of trifluoromethanesulfonic acid and sodium persulfate is 2:1:0.05:1.5:1.5. After the reaction was completed, 1,2-dichloroethane was added to quench the reaction, and the remaining acid and salt were washed away with saturated aqueous sodium bicarbonate solution, and extracted twice with 1,2-dichloromethane. The extracts were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. The residue was separated and purified by silica gel chromatography (n-hexane:ether (volume ratio 200:1)) to obtain 40 mg of the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com