Mesosulide granule and preparation method thereof
A technology of susulide and granules, which is applied in the field of mesosulide granules and its preparation, can solve the problems of poor medication compliance of patients, difficulty in taking tablets, inconvenience for patients to take, etc., and achieve good medication compliance and patient compliance. Good performance, good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
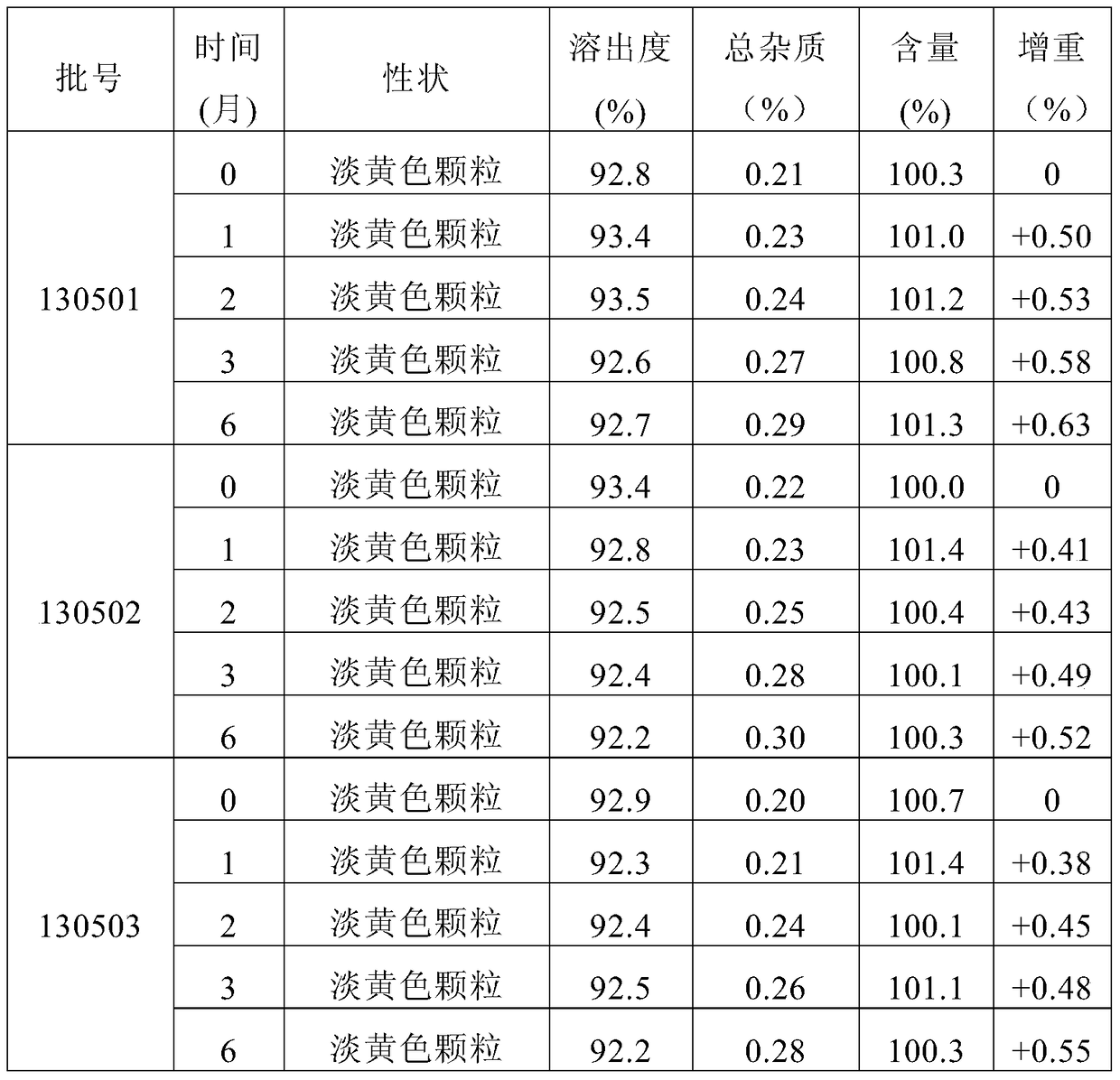
[0053] Example 1: Prescription screening and preparation process optimization of mesosulide granules
[0054] In this example, taking 50 mg mesosulide granules as an example (i.e. specification: 50 mg), the prescription and preparation process of mesosulide granules are optimized, as follows:
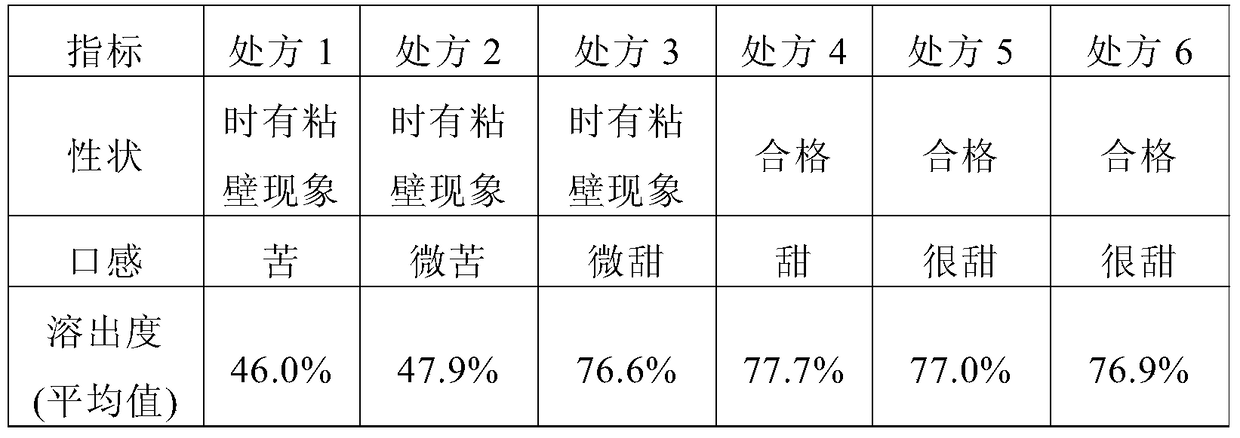
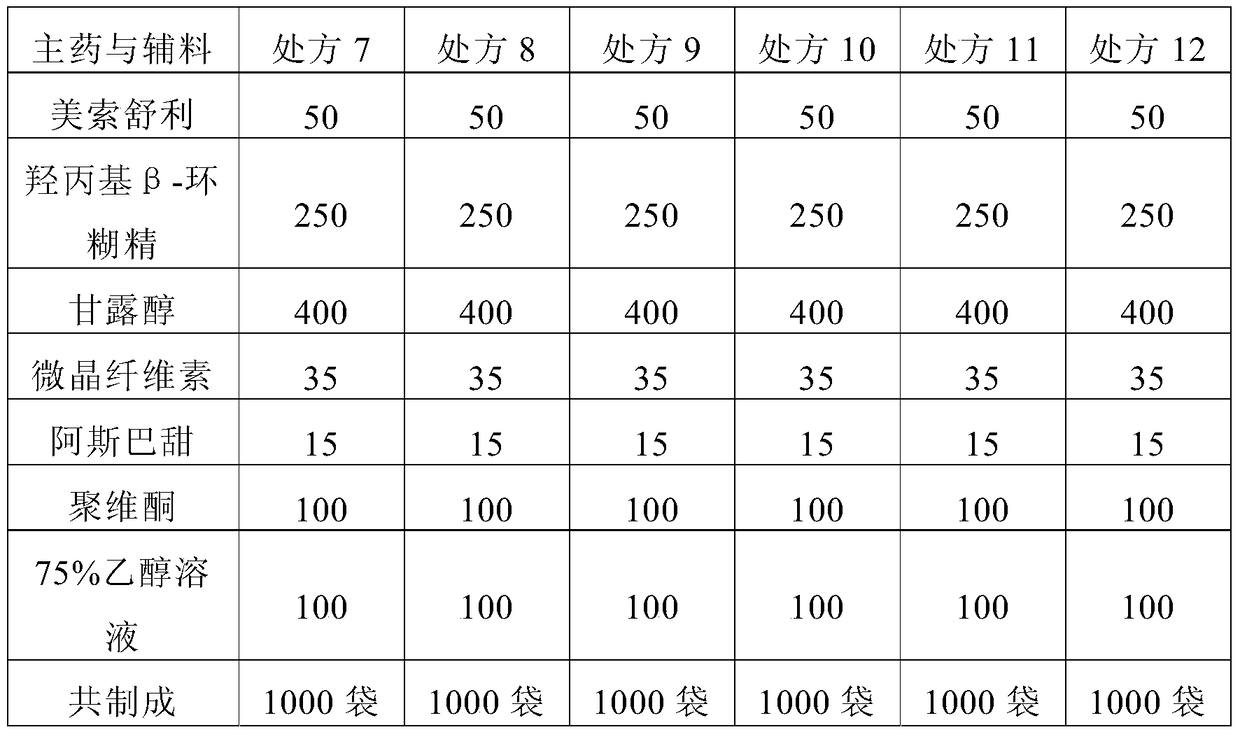
[0055] Preliminarily considering the taste, suspension, and dissolution rate of the granules, the prescription of mesosulide granules is involved. The specific prescription is shown in Table 1, and then prepared according to the prescription shown in Table 1 and the following preparation method Mesosulide granules, and then observe and detect the properties, mouthfeel and dissolution rate of the prepared mesosulide granules according to conventional methods, wherein, the mouthfeel is tried orally by volunteers. The dissolution rate determination was carried out in a pH8.8 phosphate buffer solution according to the method stipulated in the Chinese Pharmacopoeia 2010 edition, and the phos...
Embodiment 2
[0089] prescription:
[0090] 25 parts by weight of mesosulide, 125 parts by weight of hydroxypropyl β-cyclodextrin, 200 parts by weight of mannitol, 17.5 parts by weight of microcrystalline cellulose, 7.5 parts by weight of aspartame, 50 parts by weight of povidone, 75 parts by weight % ethanol qs.
[0091] The preparation process is as follows:
[0092] 1) Mesosulide, hydroxypropyl β-cyclodextrin, mannitol, microcrystalline cellulose, aspartame, and povidone were all dried separately,
[0093] 2) Mix the prescribed amount of mesosulide and hydroxypropyl β-cyclodextrin, micronize once, then add mannitol to the above micronized particles and mix, and continue to micronize until the particle size is 10 microns (μm) particles,
[0094] 3) passing microcrystalline cellulose, aspartame and povidone through an 80-mesh sieve for subsequent use;
[0095] 4) Mix the raw and auxiliary materials in the above steps 2 and 3 evenly, add 75% ethanol solution to make soft material, pass ...
Embodiment 3
[0101] prescription:
[0102] Mesosulide 25 parts by weight, hydroxypropyl β-cyclodextrin 25 parts by weight, mannitol 125 parts by weight, microcrystalline cellulose 15 parts by weight, aspartame 10 parts by weight, povidone 25 parts by weight, 75 parts by weight % ethanol qs.
[0103] The preparation process is as follows:
[0104] 1) Mesosulide, hydroxypropyl β-cyclodextrin, mannitol, microcrystalline cellulose, aspartame, and povidone were all dried separately,
[0105] 2) Mix the prescribed amount of mesosulide and hydroxypropyl β-cyclodextrin, micronize once, then add mannitol to the above micronized particles and mix, continue to micronize until the particle size is 50 microns (μm) particles,
[0106] 3) passing microcrystalline cellulose, aspartame and povidone through an 80-mesh sieve for subsequent use;
[0107] 4) Mix the raw and auxiliary materials in the above steps 2 and 3 evenly, add 75% ethanol solution to make soft material, pass the soft material through ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com