Synthesis method of tertiary aliphatic amine
A technology for the synthesis of fatty tertiary amines, which is applied in the field of surfactant intermediates and their preparation, and the synthesis of fatty tertiary amines, which can solve the problems of low cost, high reaction temperature, and high cost of raw materials, and achieve complete reaction and simple synthesis process , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of N,N-dimethyl-(9 or 10-phenyl) heptadecyl tertiary amine
[0039] N,N-Dimethyl-(9 or 10-phenyl)heptadecyl tertiary amine
[0040]
[0041] (wherein, m=5, n=1 or 2, j=8 or 7, m+n+j=14) synthesized according to the following steps:
[0042] (1) At 15°C, mix 9 or 10-phenyl-octadecanoic acid amide (methanol solution) with 7.5 mL sodium hypochlorite solution (active chlorine ≥ 5.2%, NaOH content 7-8%) in a molar ratio of 1:1.5 The reaction was carried out, stirred for 1 h, then heated to 55° C., and reacted for another 4 h to generate methyl (9 or 10-phenyl)heptadecylcarbamate.
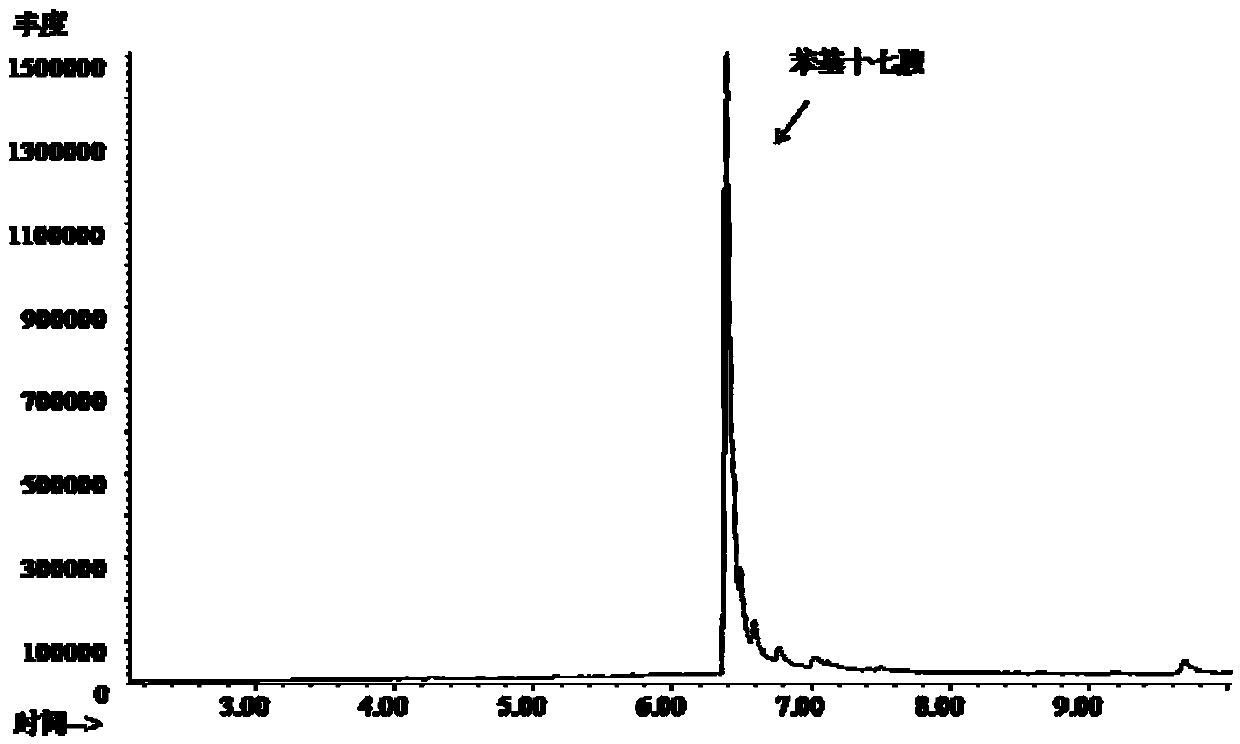
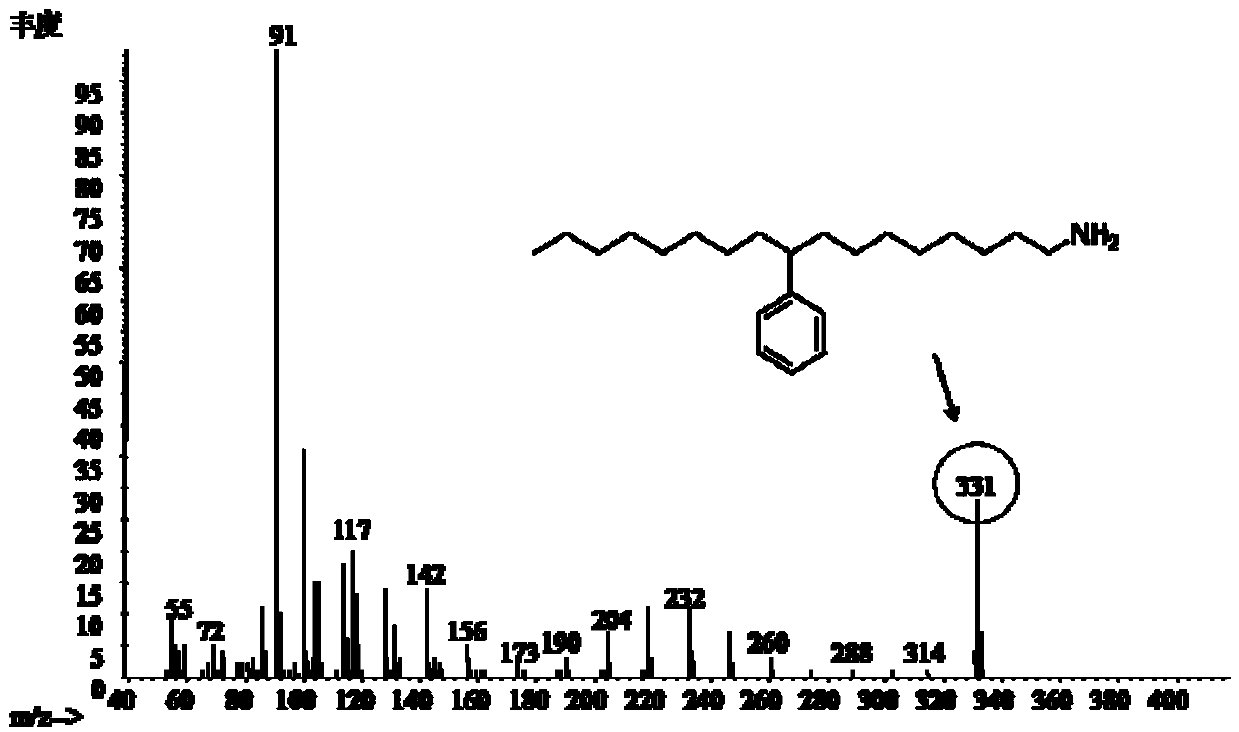
[0043] (2) Add 3.6 g of sodium hydroxide (4.5 mol / L) to the methanol solution of methyl (9 or 10-phenyl) heptadecylcarbamate, and react at 85°C for 8 h. After the reaction, the excess is removed by evaporation. of methanol, then add an appropriate amount of water, and separate the upper layer material to obtain (9 or 10-phenyl) heptadecylamine. Its GC-MS spectrum is shown ...
Embodiment 2
[0045] Example 2 Synthesis of N,N-dimethyl-(9 or 10-phenyl) heptadecyl tertiary amine
[0046] (1) At 15°C, mix the methanol solution of 9 or 10-phenyl-octadecanoic acid amide with 7.5 mL of sodium hypochlorite solution (active chlorine ≥ 5.2%, NaOH content 7-8%) in a molar ratio of 1:1.5 The reaction was carried out, stirred for 1 h, then heated to 55° C., and reacted for another 4 h to generate methyl (9 or 10-phenyl)heptadecylcarbamate.
[0047] (2) Add 3.6 g of sodium hydroxide (4.5 mol / L) to the methanol solution of methyl (9 or 10-phenyl) heptadecylcarbamate, and react at 85°C for 8 h. After the reaction, the excess is removed by evaporation. The methanol was added to the appropriate amount of water, and the upper layer was separated to obtain (9 or 10-phenyl) heptadecylamine.
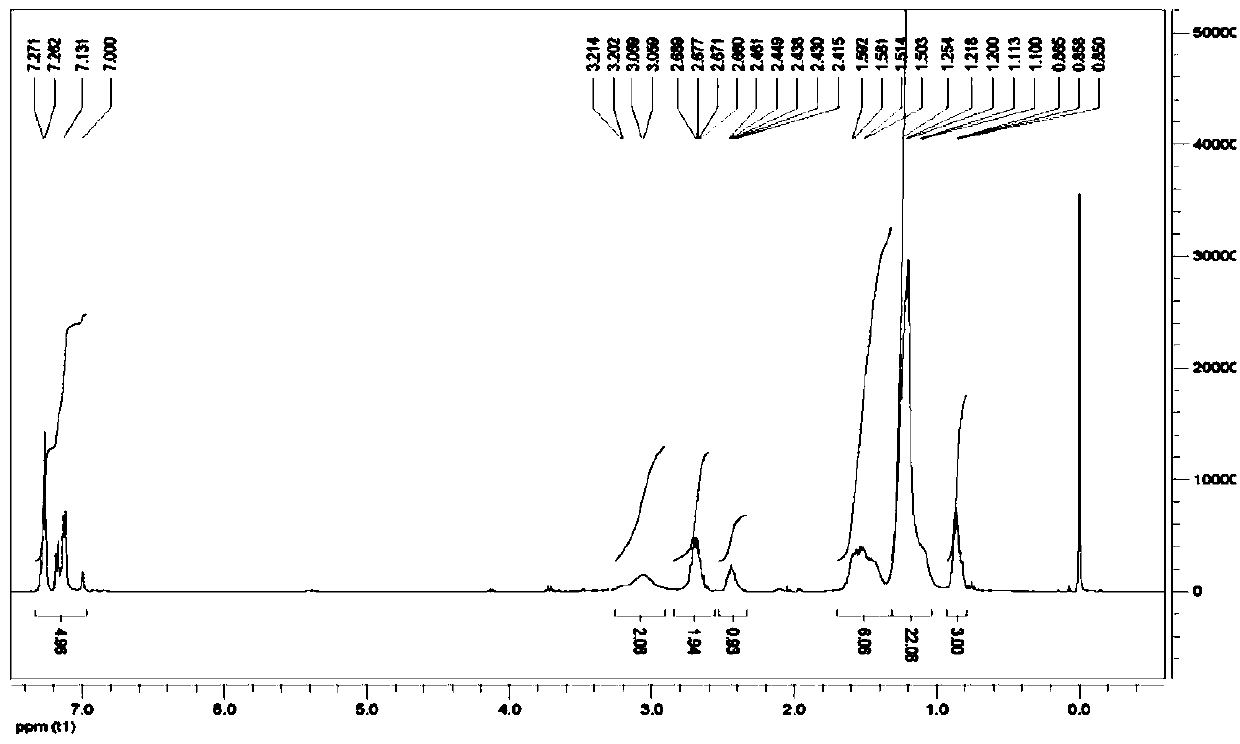
[0048] (3) Mix (9 or 10-phenyl) heptadecylamine with 1.2 mL formaldehyde solution (HCHO content 37.0-40.0%), 1.8 mL glacial acetic acid and 1.04 g zinc powder in a molar ratio of 1:2:4:2 The mi...
Embodiment 3
[0049] Example 3 Synthesis of N,N-dimethyl-(9 or 10-phenyl) heptadecyl tertiary amine
[0050] (1) At 15°C, mix 9 or 10-phenyl-octadecanoic acid amide (methanol solution) with 7.5 mL sodium hypochlorite solution (active chlorine ≥ 5.2%, NaOH content 7-8%) in a molar ratio of 1:1.5 The reaction was carried out, stirred for 1 h, then heated to 55° C., and reacted for another 4 h to generate methyl (9 or 10-phenyl)heptadecylcarbamate.
[0051] (2) Add 3.6 g of sodium hydroxide (4.5 mol / L) to the methanol solution of methyl (9 or 10-phenyl) heptadecylcarbamate, and react at 85°C for 8 h. After the reaction, the excess is removed by evaporation. The methanol was added to the appropriate amount of water, and the upper layer was separated to obtain (9 or 10-phenyl) heptadecylamine.
[0052] (3) Mix (9 or 10-phenyl) heptadecylamine with 1.2 mL formaldehyde solution (HCHO content 37.0-40.0%), 1.8 mL glacial acetic acid and 1.04 g zinc powder in a molar ratio of 1:2:4:2 The mixture was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com