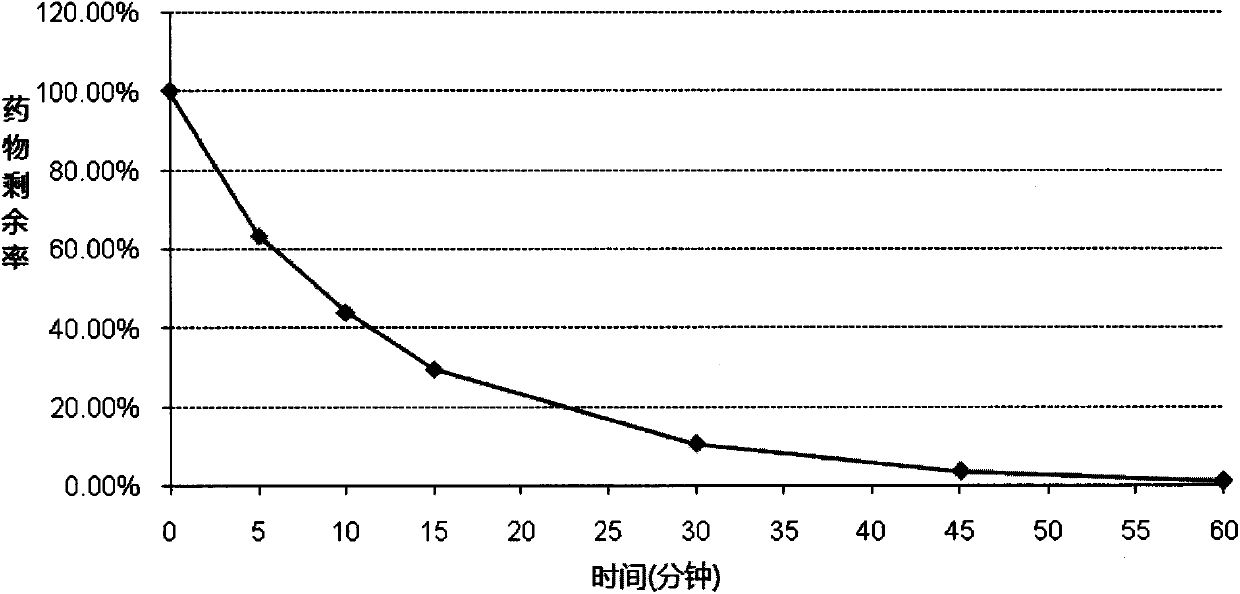
Pyrrolidinium derivatives as m3 muscarinic receptor antagonists and their use in pharmacy
A use and pharmaceutical technology, applied in the field of urological diseases or gastrointestinal diseases, the preparation of prevention or treatment of respiratory diseases, can solve the problems of long-lasting systemic side effects, uncertainty of efficacy and safety, etc., to reduce side effects risk, ensuring the efficacy of the drug, and the effect of high-efficiency antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 3R-1-methyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidine
[0037] Dissolve methyl 2,2-dithienylglycolate (1.0g, 3.9mmol) in 25mL of anhydrous toluene, add 3R-3-hydroxyl-1-methylpyrrolidine (465mg, 4.6mmol), and heat up to 120 ℃, sodium hydrogen (85mg, 2.1mmol) was added in 3 batches, and reacted for 2h. The reaction solution was extracted three times with 2N hydrochloric acid, the combined aqueous layers were washed with a small amount of ethyl acetate, the aqueous layer was adjusted to alkaline with solid sodium carbonate (until no bubbles were released), the aqueous layer was extracted three times with ethyl acetate, and the organic layers were combined , the organic layer was washed with 1N sodium hydroxide solution and saturated brine respectively, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain a yellow solid (450mg, 35%), melting point: 107°C-109°C, [α] D 25 =-7.5(c=0.42, MeOH); 1 H NMR (DMSO, 500MHz) δ7.47(m, 2H), 7.26(s, 1H), 7...
Embodiment 2
[0039] 3R-1,1-Dimethyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidine bromide (compound of formula I)
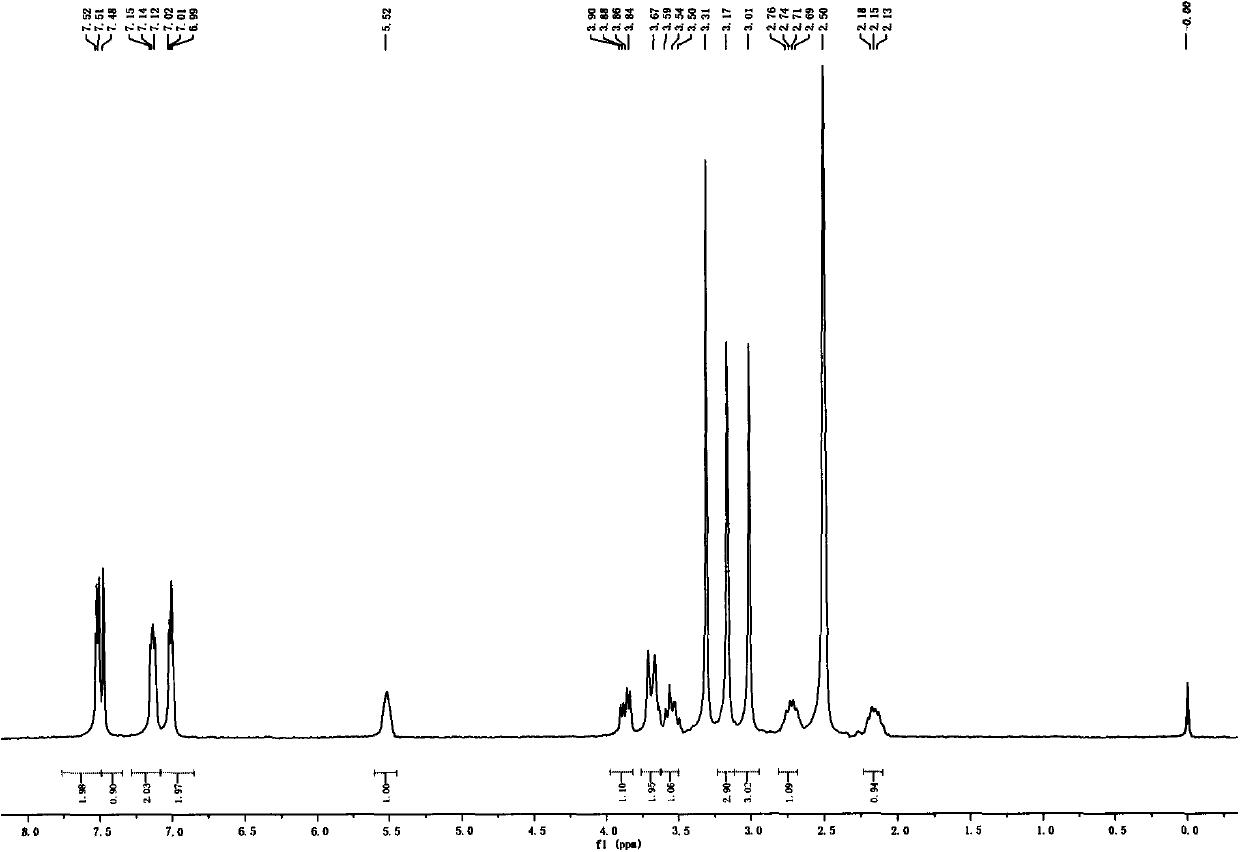
[0040] Dissolve 3R-1-methyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidine (100mg, 0.31mmol) in 0.4mL butanone, add dropwise under ice-cooling Methyl bromide (58.8mg, 0.62mmol) was slowly warmed up to room temperature and reacted overnight. Low-boiling substances were evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (dichloromethane-methanol, 40:1) to obtain an off-white solid (98 mg, 75%), [α] D 20 =-7(c=0.1, MeOH); 1 H NMR (DMSO, 500MHz) δ7.51(m, 2H), 7.48(s, 1H), 7.13(m, 2H), 7.00(m, 2H), 5.52(m, 1H), 3.93(m, 1H) , 3.73(m, 2H), 3.63(m, 1H), 3.20(s, 3H), 3.03(s, 3H), 2.74(m, 1H), 2.16(m, 1H); 13 C NMR (DMSO, 125MHz) δ170.33, 146.63, 126.69, 126.24, 125.75, 125.70, 76.24, 73.85, 69.09, 63.94, 52.66, 51.91, 29.74; m / z: 338.1 [M-Br] + ; HRMS for C 16 h 20 NO 3 S 2 Br-Br calcd338.0885, found338.0888.
Embodiment 3
[0042] 3S-1-methyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidine
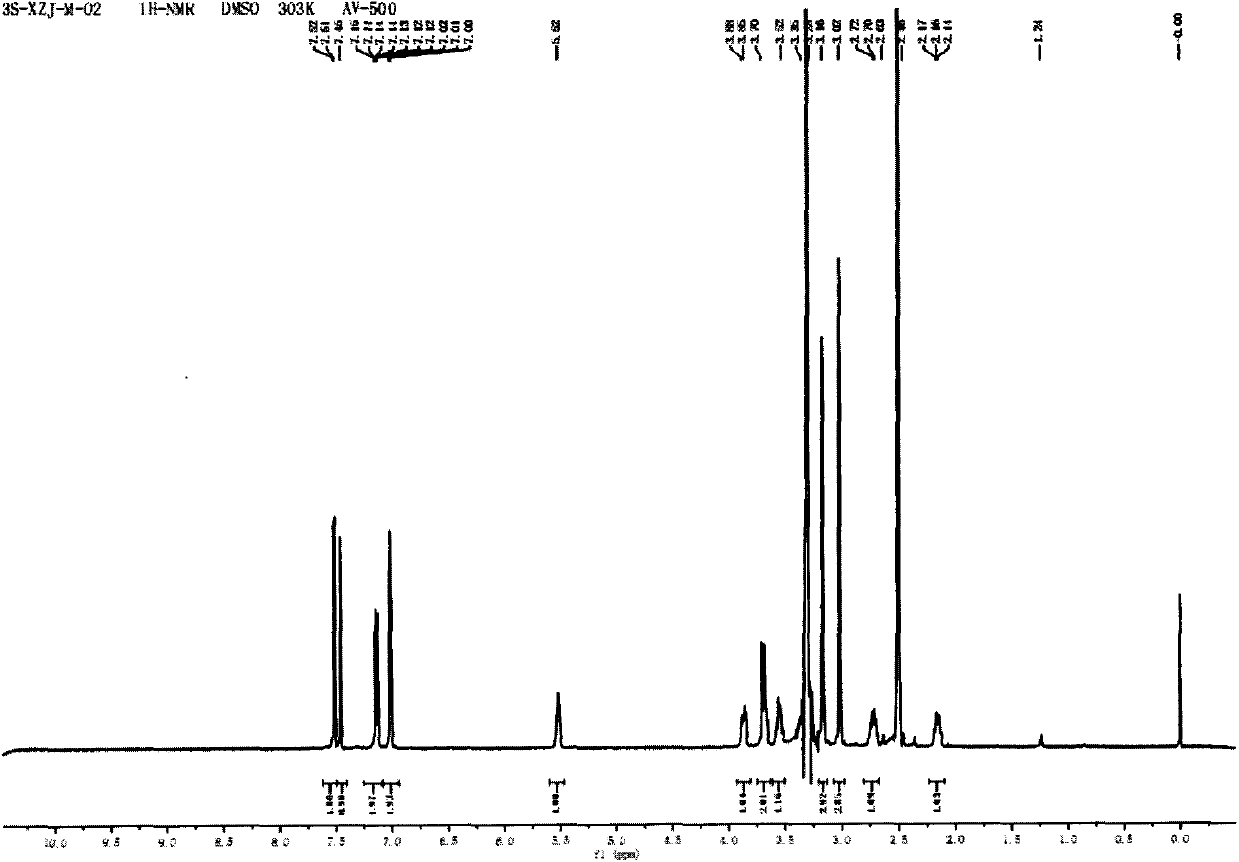
[0043] Dissolve methyl 2,2-dithienylglycolate (0.5g, 1.9mmol) in 13mL of anhydrous toluene, add 3S-3-hydroxyl-1-methylpyrrolidine (233mg, 2.3mmol), and heat up to 120 ℃, sodium hydrogen (33mg, 0.8mmol) was added in 3 batches, and reacted for 2h. The reaction solution was extracted three times with 2N hydrochloric acid, the combined aqueous layers were washed with a small amount of ethyl acetate, the aqueous layer was adjusted to alkaline with solid sodium carbonate (until no bubbles were released), the aqueous layer was extracted three times with ethyl acetate, and the organic layers were combined , the organic layer was washed with 1N sodium hydroxide solution and saturated brine respectively, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain a yellow solid (217 mg, 34%), melting point: 107 ° C ~ 109 ° C, [α] D 25 =+7.7(c=0.42, MeOH); 1 H NMR (DMSO, 500MHz) δ7.47(m, 2H), 7.26(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com