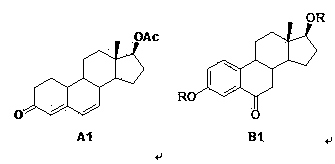
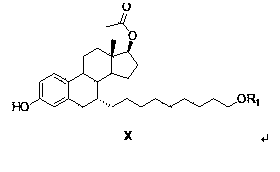
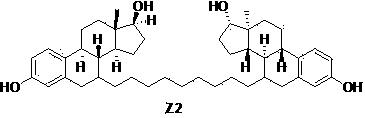
Preparation method of fulvestrant intermediate
A technology for fulvestrant and an intermediate, which is applied in the field of medicinal chemical synthesis, can solve the problems such as the avoidance and removal methods are not given, it is difficult to industrialize production, the cost is lower, and the like. Purification difficulty and the effect of reducing the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Purification of 1,9-nonanediol:
[0065] Heat and melt 1 kg of industrial-grade 1,9-nonanediol, wash it with water four times, get 673 g of 1,9-nonanediol that meets the requirements, recover 297 g of unqualified 1,9-nonanediol, The loss of material in water is 30 g, and the total utilization rate of raw material is 97%.
[0066] GC: before washing: 1,8-octanediol content: 0.2143%;
[0067] After washing: 1,8-octanediol content: 0.0573%;
[0068] Recovered sample: 1,8-octanediol content: 0.2971%;
[0069] Water loss material: 1,8-octanediol content: 0.4182%;
Embodiment 2
[0071] Preparation of 9-bromo-1-nonanol (compound 2):
[0072] Add 1,9-nonanediol (1 kg) to toluene (20 L), add hydrobromic acid (40%, 2.5 L) with stirring, and heat to reflux (internal temperature 90-100°C) for 24 hrs. Stop heating, cool to 40°C, add ethyl acetate (4L) and cold water (4L), stir and separate layers, the organic phase is washed with saturated sodium bicarbonate aqueous solution and aqueous solution successively, dried with anhydrous sodium sulfate, and spin-dried to obtain 1.6 kg Add 4.8 L of n-hexane to the crude product, stir and heat to dissolve, cool and crystallize to obtain 1.1 kg 9-bromo-1-nonanol.
[0073] GC: before crystallization: product: 95.2033%, 1,9-dibromononane: 1.9985%;
[0074] After crystallization: Product: 98.8413%, 1,9-Dibromononane: None.
Embodiment 3
[0076] Preparation of compound 3:
[0077] Add 9-bromo-1-nonanol (1.1 kg), N,N-dimethylformamide (2.86 L), imidazole (363 g) into a 10 L reaction flask, add dimethyl tert- Butylchlorosilane (772 g), the temperature is lower than 20 °C, react for 1 hour, add water (5 L), toluene (1 L) in turn, stir, separate the liquids, extract the aqueous phase with toluene (1 L), mix the organic phase, the organic phase was washed with water (1L), dried over anhydrous sodium sulfate, and spin-dried under reduced pressure at 80°C to obtain 1.56 kg of product, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com