Catalyst compositions and their use for hydrogenation of nitrile rubber
A technology of catalysts and compositions, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of low hydrogenation catalysts and uncontrollable use of catalysts, etc. problem, to achieve the effect of high hydrogenation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0410] The preparation of the novel catalyst compositions can be carried out in the presence or absence of a suitable solvent which does not deactivate the catalyst used and which does not adversely affect the hydrogenation in any other way. Preferably, an organic solvent is used to dissolve the complex catalyst. More preferred solvents include, but are not limited to, dichloromethane, benzene, toluene, methyl ethyl ketone, acetone, tetrahydrofuran, tetrahydropyran, dioxane, cyclohexane, and chlorobenzene. Particularly preferred solvents are chlorobenzene and methyl ethyl ketone. Typically, a vinyl compound is added to the solution of the complex catalyst.
[0411] Formation of the novel catalyst composition takes place prior to introducing hydrogen into the reaction system.
[0412] Step b) of the method according to the invention:
[0413] Thereafter, the hydrogenation of the nitrile rubber is carried out by contacting the nitrile rubber with hydrogen and the catalyst c...
example
[0494] Catalysts used in the examples:
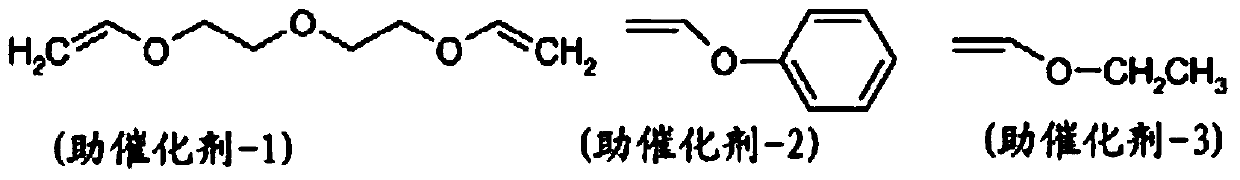
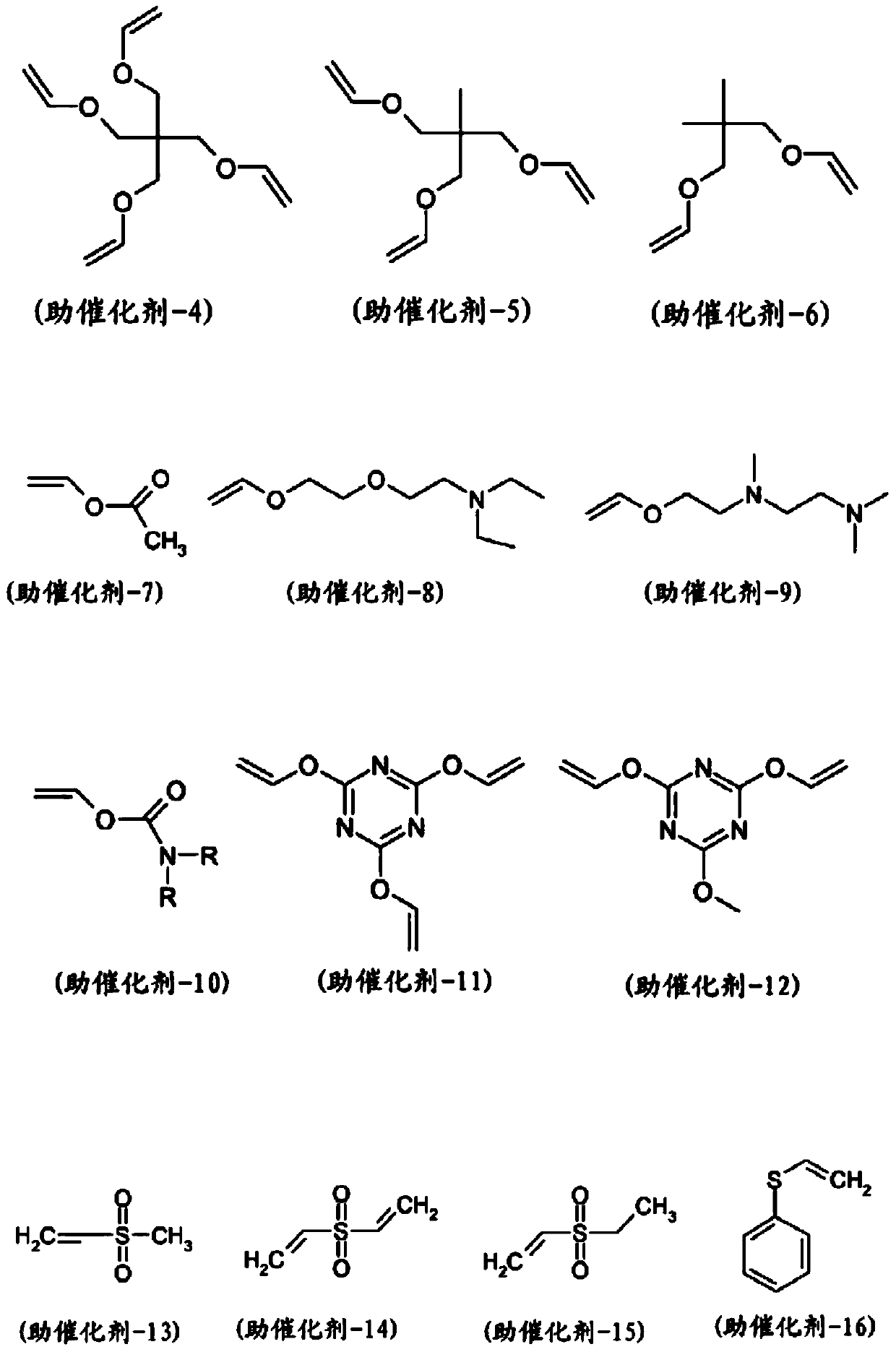
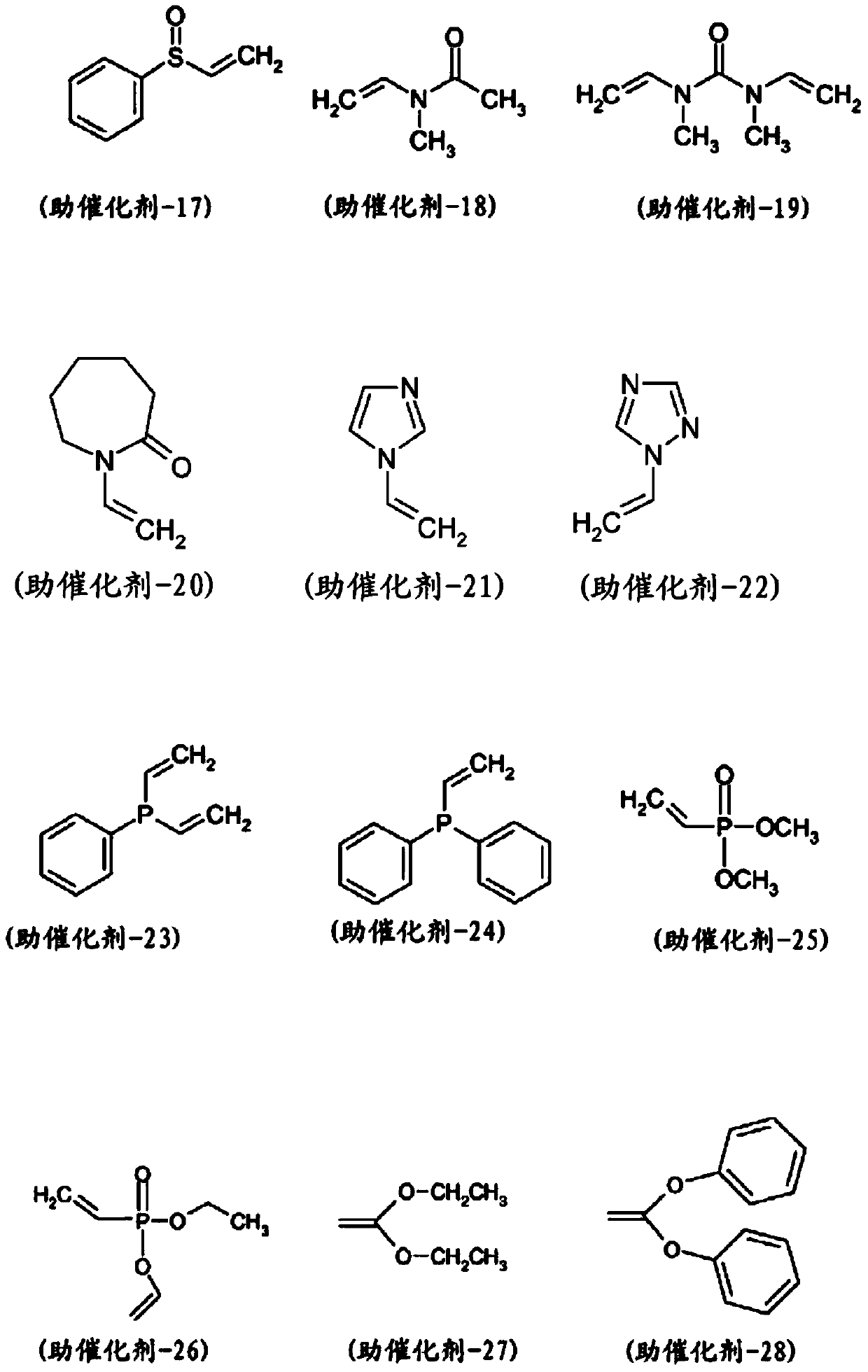
[0495] Catalysts (1) to (3) were purchased from Sigma Aldrich or Strem Chemicals Inc. Catalyst (4) was purchased from Xian Kaili Co. (China). The structures of these catalysts are shown below, where "Mes" refers to mesityl (2,4,6-trimethylphenyl) and "Cy" refers to cyclohexyl:
[0496]
[0497] These catalysts have the following molecular weights:
[0498] catalyst
molecular weight
[g / mol]
(1)
822.96
(2)
848.97
(3)
885.55
(4)
925.22
[0499] Nitrile rubber used in the examples:
[0500] The nitrile rubber used in these examples had the properties summarized in Table 1.
[0501] Table 1: Nitrile rubber (NBR) used (“CAN” means acrylonitrile)
[0502]
[0503]
[0504] Vinyl ethyl ether (VEE) was purchased from Sigma-Aldrich.
[0505] analysis test:
[0506] GPC test: the apparent molecular weight Mn and Mw are determined by Worcester (Waters) GPC ...
example 1
[0511] Example 1: (comparative example, using catalyst (4))
[0512] 18g 3431VP at 282g MCB ( The solution in 3431VP at a concentration of 6 wt%) was bubbled with nitrogen for 30 minutes in a 600 mL Parr autoclave and then heated to 120°C. Will Wilkinson catalyst (15mg) and PPh 3 (18 mg) was dissolved into another 22 g of degassed MCB and then added to the reactor. The hydrogenation was carried out at a hydrogen pressure of 4.137 MPa and a stirring speed of 800 rpm. Samples were taken from the reactor at intervals for FT-IR analysis to determine the degree of hydrogenation. After 5 hours of hydrogenation, the degree of hydrogenation reached 90.3%, the reactor was cooled to room temperature and the pressure was released. Final molecular weight and PDI are: Mn = 76,286, Mw = 260,572, PDI = 3.42.
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com