Process for the preparation of beta-C-aryl glucosides
An aryl and biaryl technology, applied in the field of preparation of β-C-aryl glucoside, can solve the problems of difficult and dangerous large-scale operation, oxidant corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
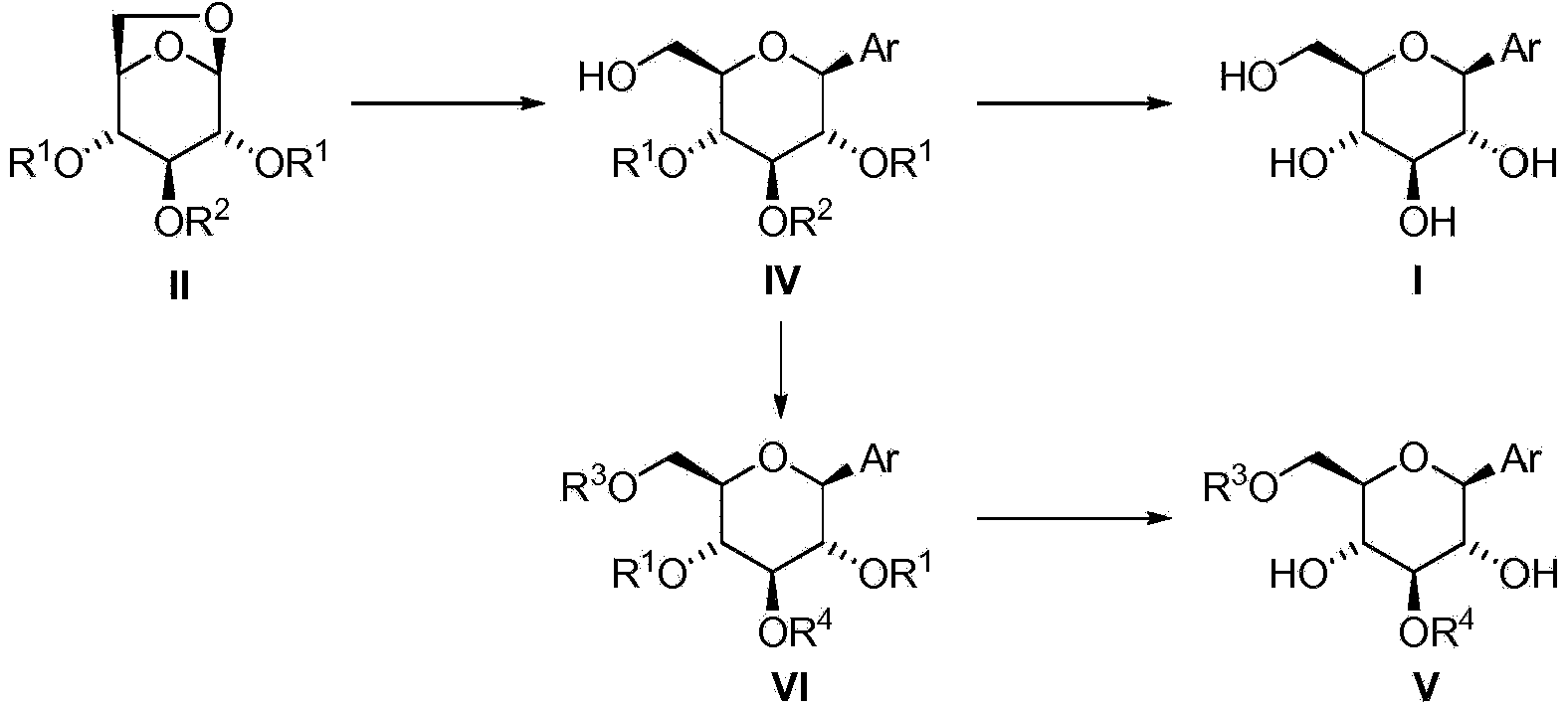
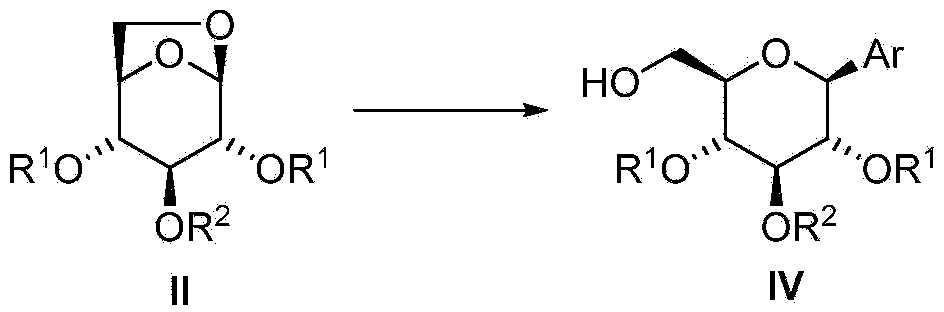
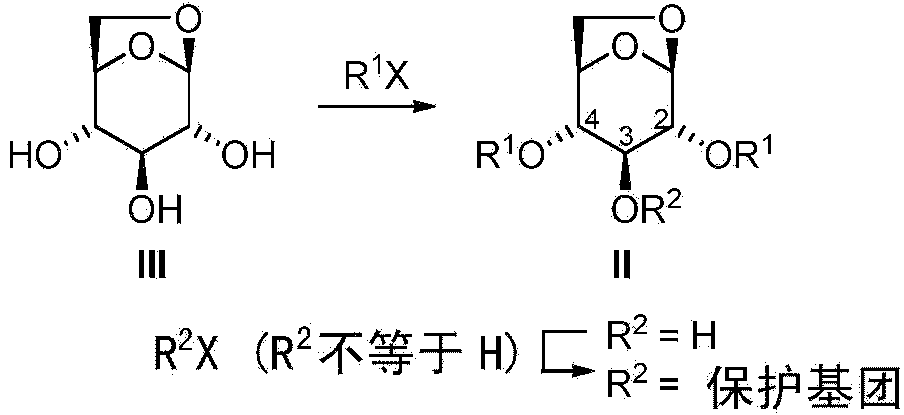
[0101] In some embodiments, the compound of formula I is provided by: i) using Me 3 The compound of formula III was pretreated with Al in MeCN (as solvent) or with DIBAL in PhMe (as solvent), and the resulting mixture was then mixed with Ar 3 Al (where Ar is aryl) exposure, optionally followed by addition of AlCl 3 As Lewis acid, or ii) compound of formula III with excess metalated aryl compound [Ar in the absence of additional Lewis acid in a suitable solvent n m 1 Y 1 p ] M 2 q immediate response. The arylation reaction of the compound of formula III is carried out above 80°C, preferably between 110°C and 180°C, most preferably at about 130°C. This arylation method without hydroxy protection is very economical in terms of the number of synthetic steps. In an approach where the cost of arylation reagents is not too high, this can be a cost- and time-competitive synthetic method for the preparation of β-C-arylglucosides since no protection and deprotection steps are re...
Embodiment
[0183] The symbols, conventions and abbreviations used in the above specification and in the examples below are consistent with those used in contemporary scientific literature such as: Journal of the American Chemical Society and The ACS Style Guide: effective communication of scientific information, pp. 3 Ed.; Coghill, A.M. and Garson, L.R., eds.; Washington, DC, Oxford University Press, New York Oxford, 2006. Except during water treatment, all experiments below were performed in an oxygen-free environment under strictly dry conditions, meaning anhydrous solvents (usually dried with molecular sieves), oven-dried glassware (dried at about 105°C) And the syringe is used under dry nitrogen atmosphere. Mix commercially available or in-house prepared organometallic reagents with AlCl before use 3 solution was titrated to determine its concentration.
[0184] Bu 2 O-di-n-butyl ether
[0185] Bu-butyl
[0186] t-Bu-tert-butyl
[0187] n-BuLi-n-butyllithium
[0188] t-BuLi-te...
Embodiment 2
[0239] Example 2 - 2,4-Di-O-tert-butyldiphenylsilyl-1-C-phenyl-β-D-glucopyranoside (IVa")
[0240]
[0241] AlCl 3 (4.0mL, 2.0mmol, 0.5M solution in THF) and phenylmagnesium bromide (1.9mL, 5.0mmol, in Et 2 2.6M solution in O) combined to give a black solution. After stirring at ambient temperature for 1 h, the solvent was evaporated under vacuum (50 Torr) followed by the addition of PhMe (6.0 mL). To 1,6-anhydro-2,4-di-O-tert-butyldiphenylsilyl-β-D-glucopyranose (0.64 g, 1.0 mmol) in PhMe (3.0 mL) at ambient temperature Add phenylmagnesium bromide (0.4mL, 1.0mmol, in Et 2 2.6 M solution in O) and after stirring for about 5 min, the mixture was then partially concentrated under reduced pressure (50 Torr) to remove Et 2O. The PhMe solution of the remaining 1,6-anhydro-2,4-di-O-tert-butyldiphenylsilyl-β-D-glucopyranose was added to the previously prepared aluminum mixture, followed by washing with PhMe (1.0 mL) diluted. The mixture was heated at gentle reflux for 27 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com