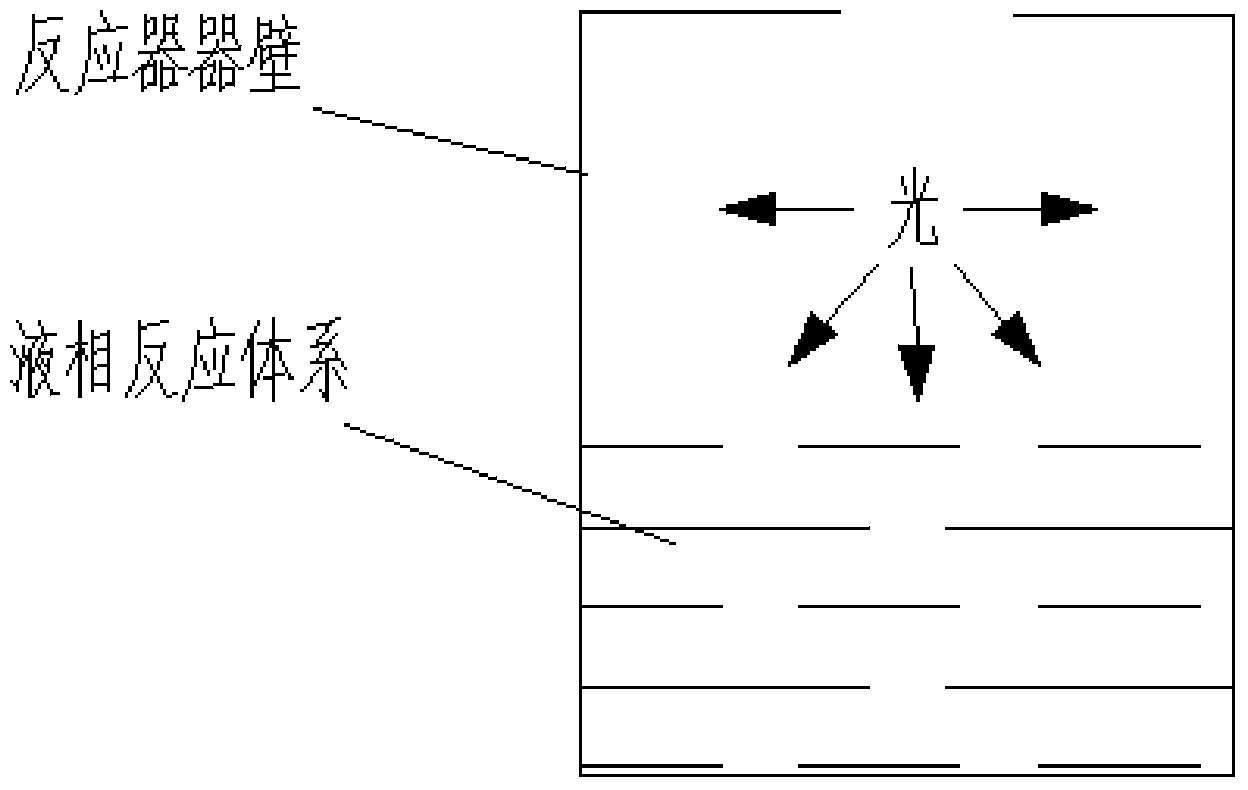
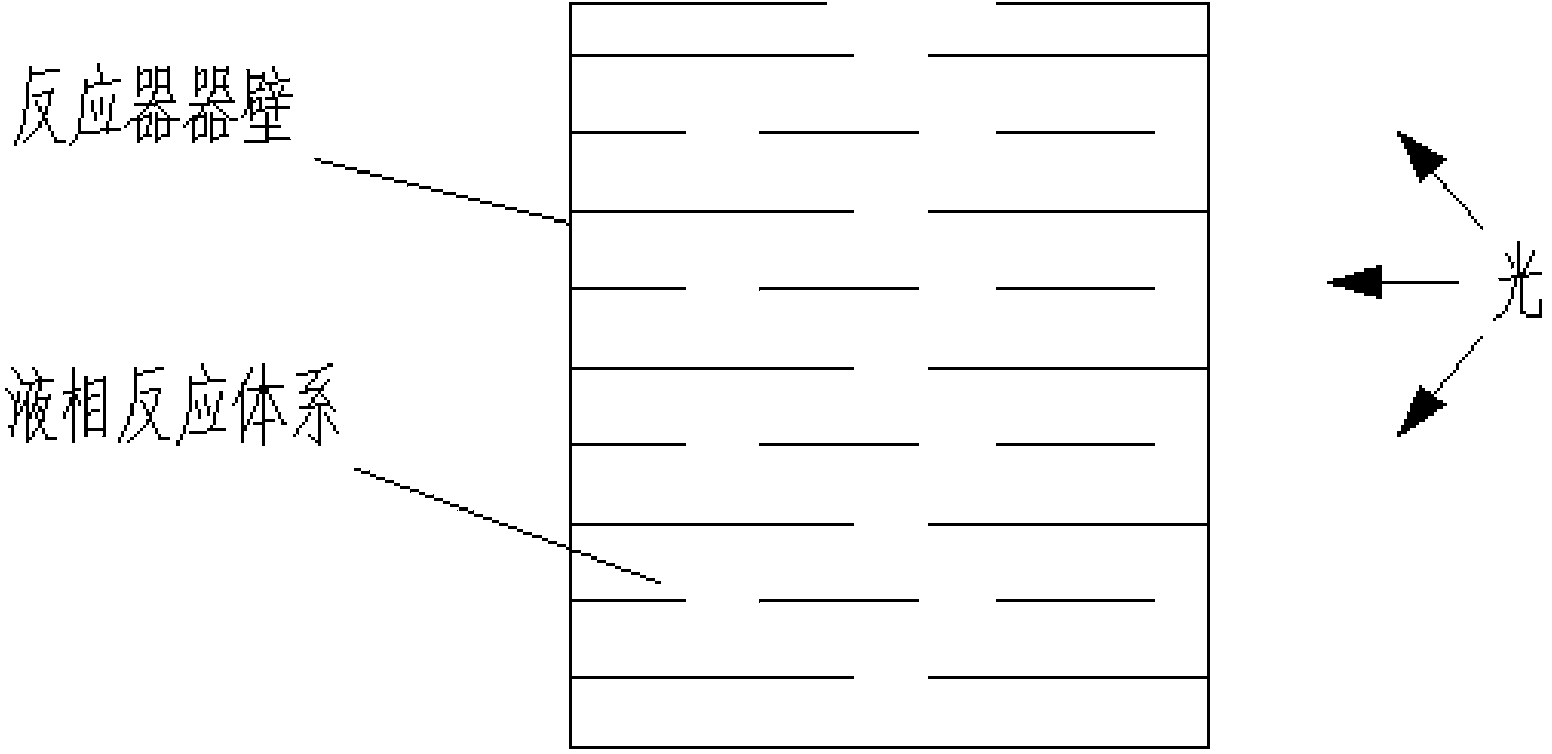
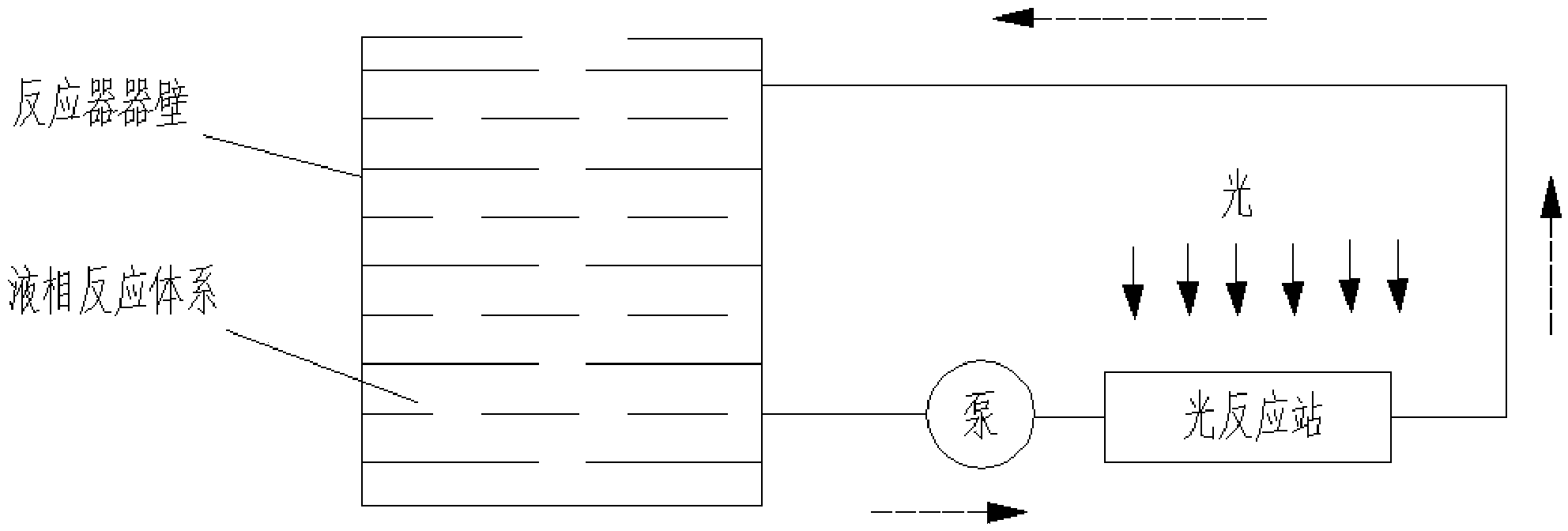
Visible light stimulated oxidation process for transforming aryl substituted vicinal diol to hydroxyketone
An oxidation process, the technology of vicinal diol, which is applied in the field of environment-friendly technology, can solve the problems such as the inability to carry out oxidation conversion of alcohols and ketones, and achieve the effect of improving production efficiency and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment one: the light-promoted Br of hydroxy ketone product A 2 / H 2 o 2 catalytic oxidation synthesis
[0040] Dissolve 166 grams of 2-methyl-1-phenylpropane-1,2-diol in 2 liters of dichloromethane, add water to adjust the volume of the aqueous phase to about 1.4 liters, and place a fluorescent lamp outside the reaction glass flask ( External light source irradiation), add 32 grams of bromine (0.2 equivalents), then slowly add 113 grams of 30% hydrogen peroxide dropwise under rapid stirring (note cooling, keep the reaction temperature in the range of 10-25 degrees Celsius all the time. Unless special It is stated that all the following other examples are tested to maintain this temperature range), and the raw materials are all converted into product A by nuclear magnetic resonance (NMR) detection after 1 hour of light and stirring reaction.
[0041] Darkness contrast test: Dissolve 166 grams of 2-methyl-1-phenylpropane-1,2-diol in 2 liters of dichloromethane, ad...
Embodiment 2
[0043] Embodiment two: the light-promoted HBr / H of hydroxy ketone product B 2 o 2 catalytic oxidation synthesis
[0044] Dissolve 206 grams of 1-(hydroxyl (phenyl) methyl) cyclohexanol in 3 liters of dichloromethane, add water to adjust the volume of the aqueous phase to about 1.6 liters, suspend the LED lamp tube inside the reaction flask (built-in Light source irradiation, white light LED light source, the wavelength range is 395-550 nanometers), add 10% aqueous solution (0.4 equivalent) of 320 grams of HBr, then slowly add 113 grams of 30% hydrogen peroxide dropwise under rapid stirring, stir under light After 4 hours of reaction, the raw materials were all converted into product B by NMR detection.
[0045] Darkness contrast test: 206 grams of 1-(hydroxyl (phenyl) methyl) cyclohexanol was dissolved in 3 liters of dichloromethane, water was added to adjust the volume of the aqueous phase to about 1.6 liters, the LED lamp tube was removed and the reaction flask was Wrap t...
Embodiment 3
[0047] Embodiment three: the light-promoted NaBr / H of hydroxy ketone product A 2 SO 4 / H 2 o 2 catalytic oxidation synthesis
[0048] Dissolve 166 grams of 2-methyl-1-phenylpropane-1,2-diol in 2 liters of dichloromethane, and use an LED surface light source with an emission wavelength of 405 nm to irradiate (external light source irradiation, 22 cm long * 4 centimeters wide, power is 6 watts per square centimeter), adding successively a 40% aqueous solution and 25 grams of sulfuric acid containing 50 grams of sodium bromide (NaBr), adding water to adjust the volume of the aqueous phase to about 1 liter. 113 grams of 30% hydrogen peroxide was slowly added under rapid stirring, and the raw material was detected by nuclear magnetic resonance (NMR) to generate product A with a conversion rate of 96% after 2 hours of light and stirring reaction.
[0049] The above experiments clearly demonstrate that HBr generated from the in situ reaction of NaBr and acid can equally efficient...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com