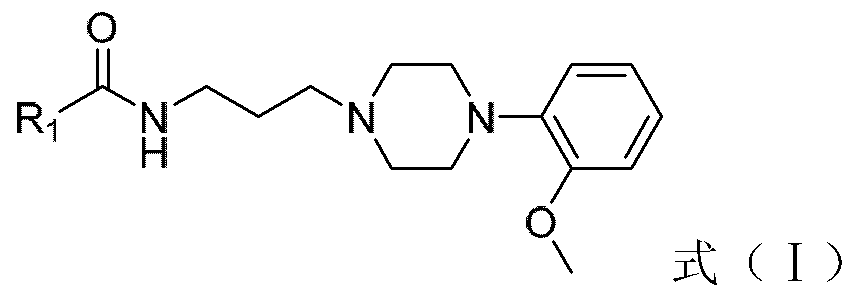
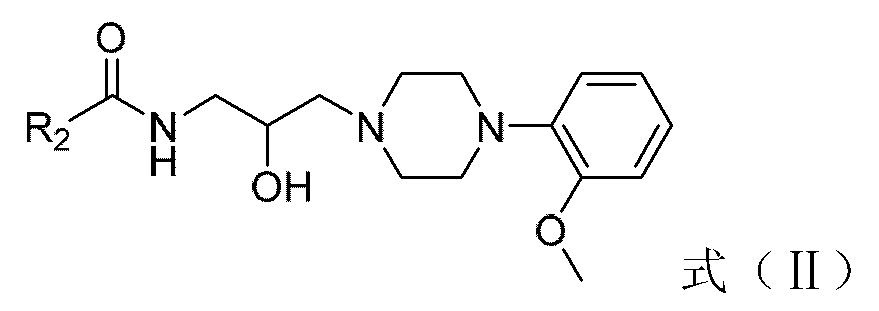
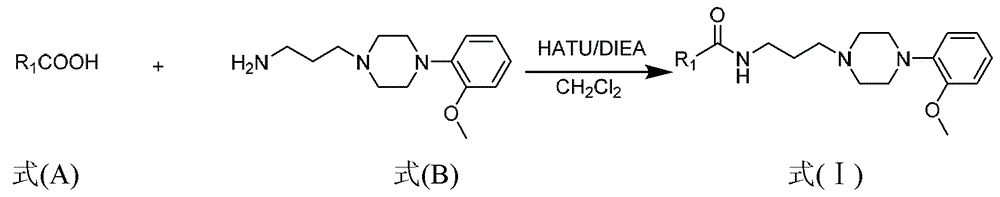
Amide-type phenylpiperazine derivative, and salt and application thereof in preparing medicine for treating benign prostatic hyperplasia
A kind of phenylpiperazine-like and derivative technology, which is applied in the application field of preparing medicine for treating benign prostatic hyperplasia, and achieves the effect of high urinary tract selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of N-(3-bromopropyl) tert-butyl carbamate
[0035]
[0036] 3-Bromopropylamine hydrobromide (5g, 22.84mmol) and di-tert-butyl dicarbonate (5.98g, 27.40mmol) were dissolved in an appropriate amount of dichloromethane and stirred at room temperature, and triethylamine (9.5ml, 68.16 mmol) and 4-dimethylaminopyridine (0.28g, 2.28mmol) in dichloromethane mixed solution. The titration time was controlled to be 1 h, and stirring was continued at room temperature for 30 min after the titration was completed. After the reaction was complete, the reaction solution was washed once with 0.5N HCl and saturated brine, the organic layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the organic solvent to obtain 4.17 g of a light yellow oil, and the obtained crude product was not further purified. used directly for the next reaction.
Embodiment 2
[0037] Example 2: Preparation of N-tert-butoxycarbonyl-3-[4-(2-methoxyphenyl)-1-piperazinyl]propylamine
[0038]
[0039] Dissolve 4.17 g of crude tert-butyl N-(3-bromopropyl)carbamate and 1-(2-methoxyphenyl)piperazine (3.70 g, 19.24 mmol) obtained in the previous step in an appropriate amount of CH 2 Cl 2 , add anhydrous potassium carbonate (2.66g, 19.24mmol) and sodium iodide (0.26g, 1.73mmol), stir at room temperature for 1d. After the reaction was completed, the reaction solution was washed once with water, anhydrous NaSO 4 Dry and concentrate under reduced pressure to obtain 7.28 g of crude milk product. The crude product was purified by 300-400 mesh silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 4:1, adding 5‰ triethylamine) to obtain 5.69 g of light yellow oil, Example 1 and Example 2 The total yield of the two-step reaction was 71.3%. ESI-MS(m / z): 350.3[M+H] + .
Embodiment 3
[0040] Example 3: 3-[4-(2-methoxyphenyl)-1-piperazinyl]propylamine
[0041]
[0042] Dissolve 500mg of N-tert-butoxycarbonyl-3-[4-(2-methoxyphenyl)-1-piperazinyl]propylamine in 40ml of dichloromethane, slowly add 10ml of trifluoroacetic acid dropwise, and stir at room temperature for 3h. After the reaction was complete, 20% NaOH solution was added to the reaction solution to adjust the pH to 13. Dichloromethane was extracted twice, and the organic layers were combined. After the organic layer was dried with anhydrous sodium sulfate, it was concentrated under reduced pressure to remove the organic solvent and a light yellow oil was obtained. The obtained product was directly used in the next reaction without further purification. ESI-MS(m / z): 2502[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com