A kind of method for synthesizing l-tert-leucine with double enzymes
A technology of tertiary leucine and trimethylpyruvic acid, which is applied in the field of double-enzyme synthesis of L-tertiary leucine, can solve the problems of high production cost and long reaction time, and achieves reduction of reaction cost, shortened reaction time and improved The effect of reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
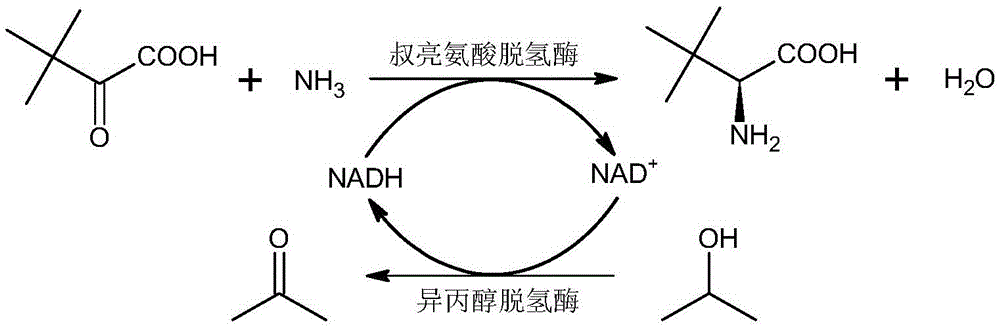
[0013] A method for synthesizing L-tert-leucine with two enzymes, configuring reaction solution A: adding 0.5 g of trimethylpyruvate, 0.47 mL of ammonia water with a concentration of 30% and 0.5 mL of isopropanol to a concentration of 0.63 M In the ammonium formate solution with a volume of 2mL, stir evenly, and adjust the pH value to 9.0; prepare reaction solution B: mix 7.5mg leucine dehydrogenase (commercially available), 7.5mg isopropanol dehydrogenase (purchased in Ningbo Madison Pharmaceutical Technology Co., Ltd. (trade name: 111EM006) and 2.5mg of coenzyme NADH (commercially available) were dissolved in water, stirred evenly, and made into 2.03mL enzyme solution; React at a reaction temperature of 40°C and a stirring rate of 400 rpm for 4 hours. After the reaction is complete, filter to obtain a solid of L-tert-leucine. The above-mentioned reacted reaction formula is as follows: figure 1 shown.
Embodiment 2
[0015] It is basically the same as Example 1, except that the mass of trimethylpyruvate is 0.25 g, the pH value is 9.5, the reaction temperature is 25° C., and the reaction time is 8 hours.
Embodiment 3
[0017] It is basically the same as Example 1, except that the mass of trimethylpyruvate is 1.0g, the pH value is 8.0, and the reaction temperature is 30°C. The reaction solution A is poured into the reaction solution B in two times: that is, the reaction solution is first Add 1.5 mL of solution A to reaction solution B, react for 3 hours, and then add the remaining amount of reaction solution A to reaction solution B for 9 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com