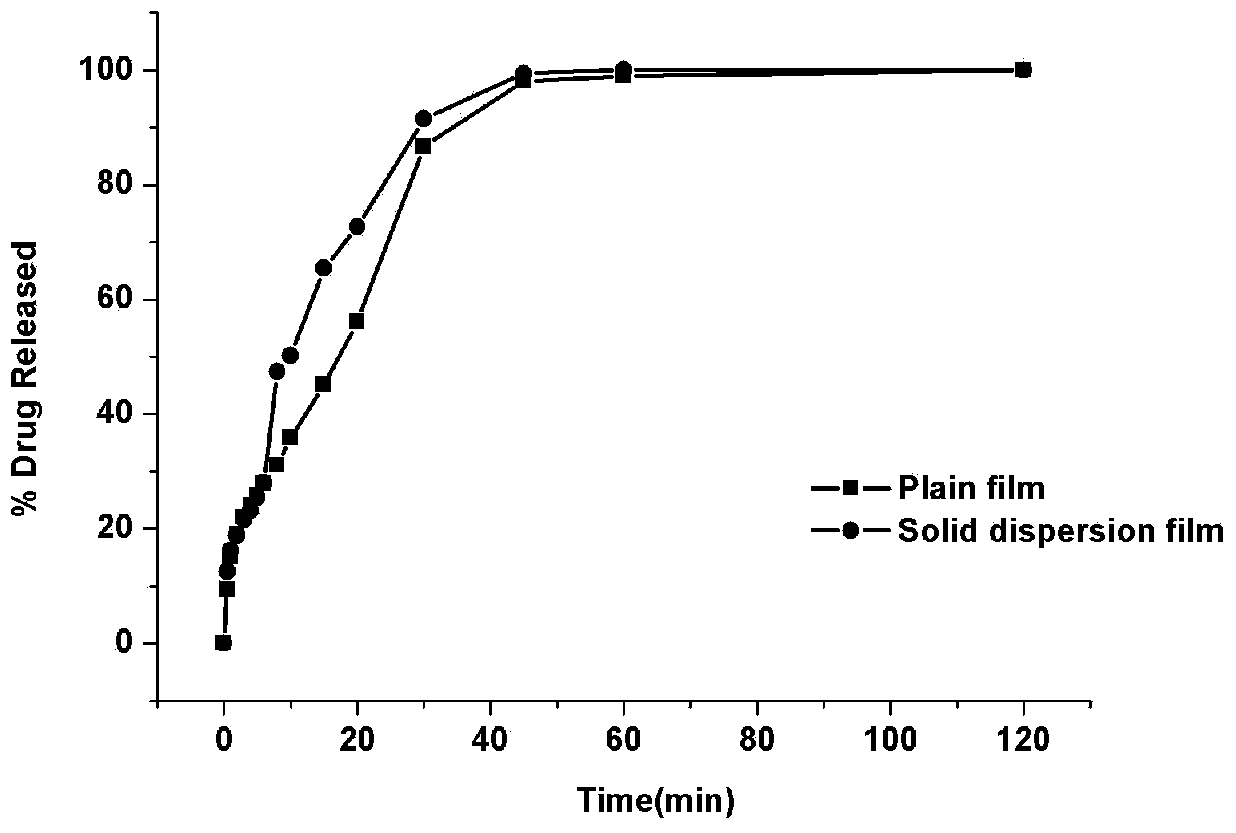
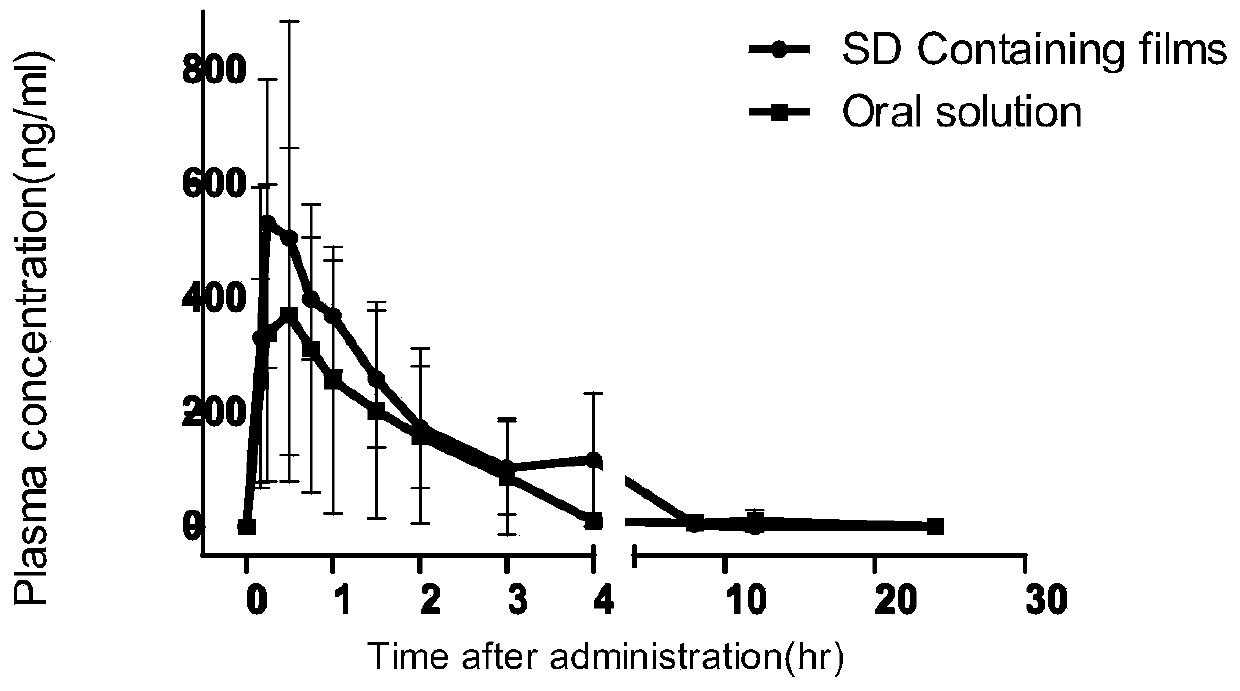
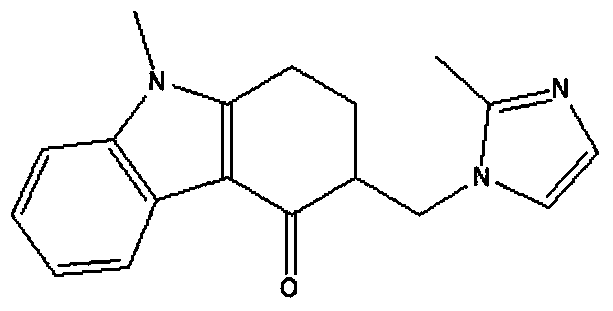
Oral quick-release film agent containing ondansetron hydrochloride solid dispersion
A technology of ondansetron hydrochloride and solid dispersion, which is applied in the field of oral immediate-release films, can solve the problems of frequent administration, poor patient compliance, and low bioavailability, so as to improve bioavailability and patient compliance, The effect of rapid drug release and convenient application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 prepares the oral immediate-release film containing ondansetron hydrochloride solid dispersion
[0031] Prepare 100 tablets, each containing 8 mg of ondansetron hydrochloride, the amount of raw materials and auxiliary materials is:
[0032] Ondansetron hydrochloride 0.8g, PVPK300.8g, hydroxypropyl methylcellulose 1.8g, methylcellulose 0.6g, xanthan gum 0.04g, PEG4000.9g, erythritol 0.1g.
[0033] The preparation method steps are as follows:
[0034] (1) Preparation of ondansetron hydrochloride solid dispersion: get ondansetron hydrochloride and polyvinylpyrrolidone, mix, place in a mortar and grind evenly, add appropriate amount of methanol (make the concentration of ondansetron hydrochloride be 4%, w / v, unit g / ml), stir evenly to make it dissolve, put it on a 40°C constant temperature water bath and stir for 1h, evaporate the solvent methanol to dryness under reduced pressure, put the product in a vacuum oven at 45°C, and dry it for 48h (to remove residu...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap