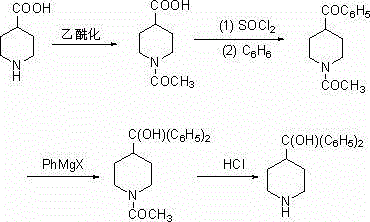
Method for synthesis of alpha,alpha-diphenyl-4-piperidine methanol
A technology of piperidinemethanol and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high production cost, and achieve the effects of low production cost, suitable for industrialization, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of α, the synthetic method of α-diphenyl-4-piperidinemethanol, the concrete steps of its synthetic process are as follows:
[0031] (1) Synthesis of N-acetyl-4-piperidinecarboxylic acid
[0032] Add 4-piperidinecarboxylic acid (38.8g, 0.30mol) and 58mL of acetic anhydride to a 250 mL four-necked flask equipped with a drying tube at the mouth of the condenser, slowly raise the temperature to reflux and continue for 6 hours, TLC detects that the reaction is complete, and the obtained The reaction solution was cooled to room temperature, a large amount of light brown solids were precipitated, the solids were filtered out with suction, and the solids were washed with an appropriate amount of methyl tert-butyl ether and then filtered with suction to obtain light brown solids, which were recrystallized with methanol to obtain 46.3 g of off-white solids. Yield 90.1%;
[0033] The amount of 4-piperidinecarboxylic acid and acetylating reagent used in the above N-acetylat...
Embodiment 2
[0050] A kind of α, the synthetic method of α-diphenyl-4-piperidinemethanol, the concrete steps of its synthetic process are as follows:
[0051] (1) Add 4-piperidinecarboxylic acid (38.8g, 0.30mol) and 32 mL of acetyl chloride into a 250 mL four-necked flask equipped with a drying tube at the mouth of the condenser, slowly raise the temperature to reflux and continue for 4 hours, TLC After the detection reaction was completed, the resulting reaction liquid was cooled to room temperature, and a large amount of light brown solids were precipitated, and the solids were filtered out with suction. The solids were washed with an appropriate amount of methyl tert-butyl ether and then filtered with suction to obtain a light brown solid, which was recrystallized with methanol to obtain a White solid 46.3g, yield 90.1%;
[0052] The amount of 4-piperidinecarboxylic acid and acetylating reagent used in the above N-acetylation reaction is calculated in molar ratio, that is, 4-piperidinec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com