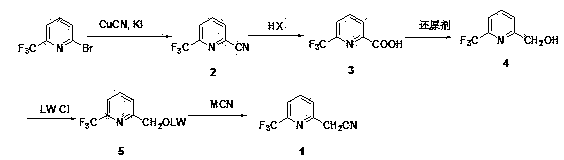
6-trifluoromethyl pyridine-2-acetonitrile synthesis method
A technology of trifluoromethylpyridine and a synthesis method is applied in the field of synthesis of pharmaceutical intermediate-6-trifluoromethylpyridine-2-acetonitrile, can solve problems such as inability to obtain, and achieves the effects of convenient operation and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1. Preparation of 2-cyano-6-trifluoromethylpyridine 2
[0019] 2-Bromo-6-trifluoromethylpyridine (1.13 g, 5 mmol), cuprous cyanide (0.67 g, 7.5 mmol) and potassium iodide (1.25 g, 7.5 mmol) were suspended in DMF (15 mL) and heated React at 130°C for 12 h. The resulting reaction mixture was poured into a mixed solvent of ice water (100 mL) and ethyl acetate (50 mL). The solid was filtered off, and the filtrate was extracted with ethyl acetate (50 mL*3). The combined organic phases were washed with saturated brine and dried over anhydrous magnesium sulfate. Concentration gave pale yellow oil 2-cyano-6-trifluoromethylpyridine ( 2 , 0.95 g). No need for purification, it can be directly used in the next step.
Embodiment 2
[0020] Example 2. Preparation of 6-trifluoromethylpyridine-2-carboxylic acid 3
[0021] A mixture of 2-cyano-6-trifluoromethylpyridine (2, 0.95 g) and concentrated hydrochloric acid HCl (37%, 15 mL) was heated at reflux for 2 hours. After cooling, a white solid 6-trifluoromethylpyridine-2-carboxylic acid ( 3 , 0.70 g, 74%. 1HNMR (DMSO-d6): 13.69 (s, 1H), 8.24-8.31 (m, 2H), 8.10-8.14 (m, 1H).
Embodiment 3
[0022] Example 3. Preparation of 6-trifluoromethyl-2-hydroxymethylpyridine 4
[0023] 6-Trifluoromethylpyridine-2-carboxylic acid (3,382 mg, 2 mmol) was dissolved in tetrahydrofuran (20 mL) and cooled to -10 °C. The reducing agent lithium aluminum tetrahydrogen LiAlH4 (152 mg, 4 mmol) was added in portions. After the mixture was stirred for 1 h, a few drops of water were added to quench the reaction. Ethyl acetate (20 mL) was added for extraction. The organic layer was separated and dried over anhydrous magnesium sulfate. Concentrate to dryness to obtain an oily crude product ( 4 , 320 mg) was used directly in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com