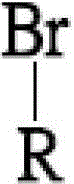Side-chain group equipped polyimide and preparation method thereof
A technology of polyimide and polyamic acid, applied in the field of polyimide with side chain group and its preparation, can solve the problem of poor solubility, large intermolecular force of polyimide, and reducing the possibility of polyimide. Processability and other issues, to achieve the effect of good solubility and low intermolecular polar force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The diamine monomer (a) of the first embodiment of the present invention is 1,4-bis(4-aminophenoxy)-2-adamantylbenzene, and its structure is as follows:
[0032]
[0033] The preparation method of the above diamine monomer includes three main steps: the formation of 2-adamantyl hydroquinone (2-adamantyl hydroquinone), the formation of 1,4-bis(4-nitrophenoxy)-2- Adamantylbenzene (1,4-bis(4-notrophenoxy)-2-adamantylbenzene) and the formation of 1,4-bis(4-aminophenoxy)-2-adamantylbenzene (1,4-bis( 4-aminophenoxy)-2-adamantyl benzene).
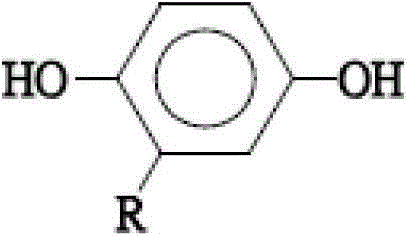
[0034] The steps for forming 2-adamantyl hydroquinone include, first, add 15 grams (69.77 mmol) of 1-bromoadamantane, 15.35 grams (139.5 mmol) of hydroquinone, and 75 ml of benzene into a 250 ml three-necked flask purged with nitrogen In, heating to reflux for 72 hours, the reaction temperature is about 80-85°C, the boiling point of benzene. It should be noted that during the reaction process, a large amount of white smoke can be seen ...
Embodiment 2
[0061] The dianhydride monomer (b) of the second embodiment of the present invention is 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride, and its structure is as follows:
[0062]
[0063] The preparation method of this dianhydride monomer includes four main steps: forming 2-adamantyl hydroquinone (2-adamantyl hydroquinone), 1,4-bis(3,4-dicyanophenoxy)- 2-adamantylbenzene (1,4-bis(3,4-dicyanophenoxy)-2-adamantyl benzene), 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylbenzene ( 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantyl benzene) and 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride (1,4-bis (3,4-dicarboxyphenoxy)-2-adamantyl benzene dianhydride).
[0064] The steps for forming 2-adamantylhydroquinone are the same as those in the first embodiment, and will not be repeated here. And the step of forming 1,4-bis(3,4-dicyanophenoxy)-2-adamantylbenzene comprises, 2 grams (8.197mmol) of 2-adamantylhydroquinone, anhydrous carbonic acid Add 1.25 g (9.06 mmol) of pot...
Embodiment 3
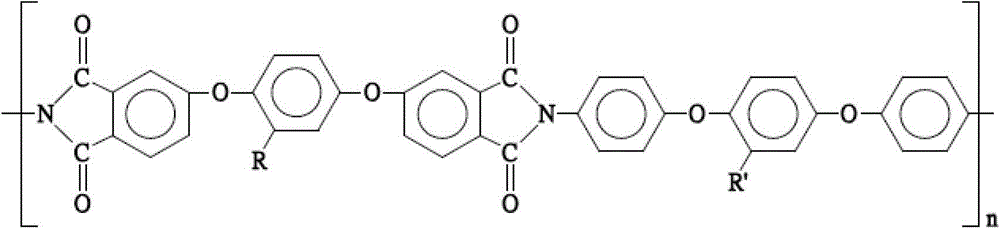
[0081] The polyimide compound (c) of the third embodiment of the present invention uses 1,4-bis(4-aminophenoxy)-2-adamantylbenzene as the diamine monomer (a) of the present invention, and 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride is used as the dianhydride monomer (b) of the present invention to prepare the polyimide compound (c). And the polyimide compound (c) of the third embodiment of the present invention has the following structure:
[0082]
[0083] In summary, the present invention provides a polyimide compound with side chain groups and a preparation method thereof. The polyimide compound has a large side chain group, so its structural symmetry and arrangement regularity are relatively low, thereby improving the solubility of the polyimide compound in various solvents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com