Chroma alumina catalysts for alkane dehydrogenation
A technology of dehydrogenation catalyst and alumina, applied in carbon compound catalysts, catalysts, catalysts for physical/chemical processes, etc., can solve the problems such as very difficult control of temperature distribution of catalytic bed, difficult control of coke distribution, influence of heat, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
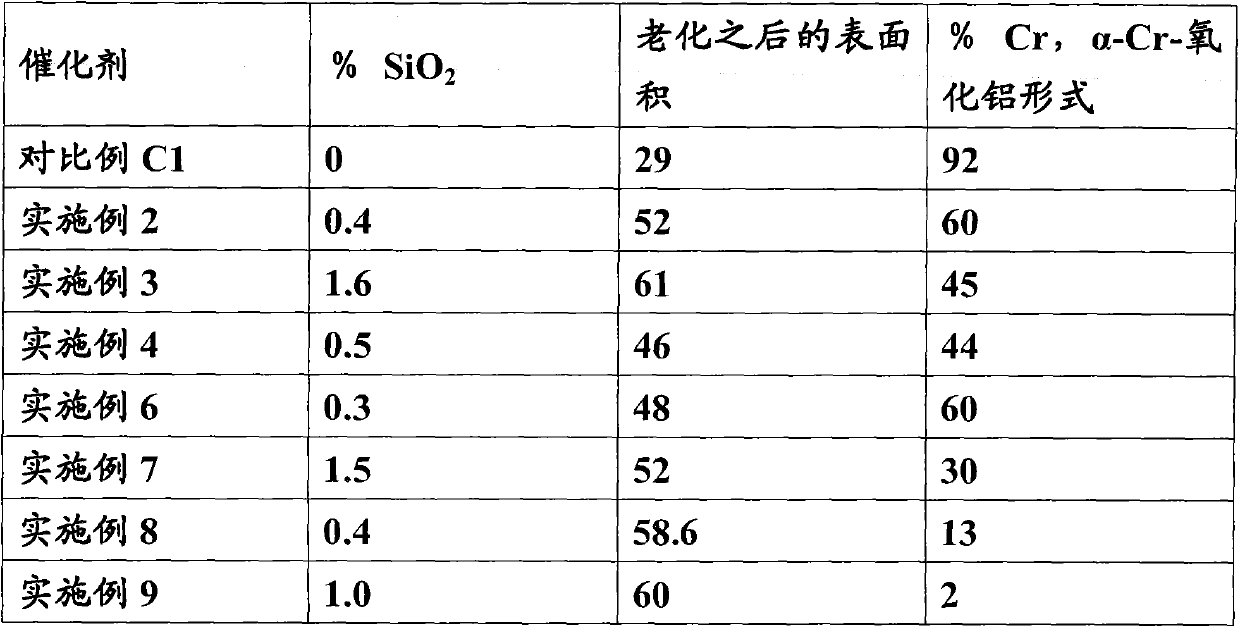
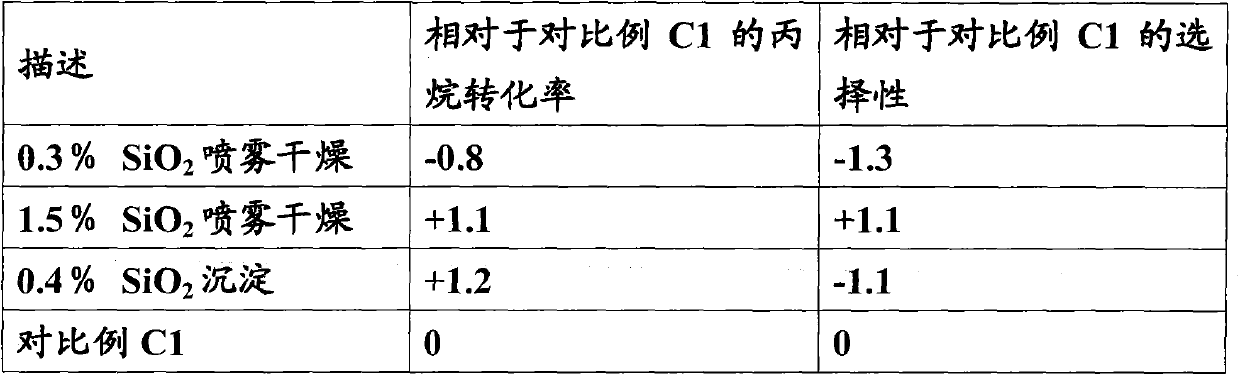
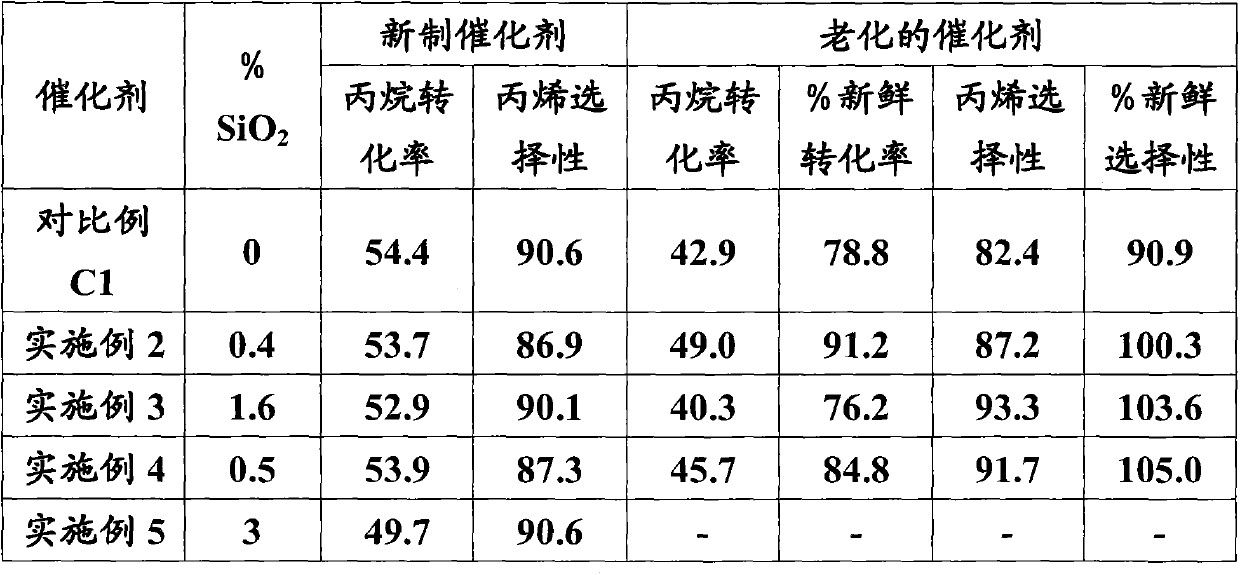
[0056] Embodiment 2 (with 0.4% SiO 2 Na-silicate impregnation)
[0057] Alumina trihydrate (2700.4 grams) was charged to 10L in the mixer. 24.5g Sodium silica (PQ; 28.7% SiO 2 ) in 80 ml of water was added to the mixer. After the mixer was running for 2 minutes, a solution comprising water (200 grams) and nitric acid (198.0 grams) was added to the mixer. The mixture was mixed for a total of about 20 minutes. The mixture was formed into cylindrical extrudates (1 / 8" diameter), dried overnight at 90 degrees Celsius, and then calcined in air at 800 degrees Celsius for 2 hours. The calcined extrudates were made without external Cool in a cooled furnace.
[0058] A portion (250 g) of the calcined alumina extrudate was impregnated with an aqueous solution of chromic acid (88 g), a sodium dichromate solution (7.7 g, 69% sodium dichromate dihydrate) and water (52 g) To first wet. The samples were dried and calcined in air at 750 degrees Celsius for 2 hours. The impregna...
Embodiment 3
[0059] Embodiment 3 (utilize 1.6%SiO 2 Na-silicate impregnation)
[0060] Alumina trihydrate (2700.4 grams) was charged to 10L in the mixer. 98.3g Sodium silica (PQ; 28.7% SiO 2 ) solution was added to the mixer. After the mixer was running for 2 minutes, a solution comprising water (200 grams) and nitric acid (198.0 grams) was added to the mixer. The mixture was mixed for a total of about 20 minutes. The mixture was formed into cylindrical extrudates (1 / 8" diameter), dried overnight at 90 degrees Celsius, and then calcined in air at 800 degrees Celsius for 2 hours. The calcined extrudates were made without external Cool in a cooled furnace.
[0061] A portion (250 g) of the calcined alumina extrudate was impregnated with an aqueous solution of chromic acid (87.7 g), sodium dichromate solution (3 g, 69% sodium dichromate dihydrate) and water (49 g) To first wet. The samples were dried and calcined in air at 750 degrees Celsius for 2 hours. The impregnated extru...
Embodiment 4
[0062] Embodiment 4 (with colloidal silicon dioxide 0.5% SiO 2 co-extrusion)
[0063] Alumina trihydrate (2700.4 grams) was charged to 10L in the mixer. 33.75 grams of colloidal silicon dioxide ( 2327) in 50 ml of water was added to the mixer. After the mixer was running for 2 minutes, a solution comprising water (290 grams) and nitric acid (132.0 grams) was added to the mixer. The mixture was mixed for a total of about 20 minutes. The mixture was formed into cylindrical extrudates (1 / 8" diameter), dried overnight at 90 degrees Celsius, and then calcined in air at 800 degrees Celsius for 2 hours. The calcined extrudates were made without external Cool in a cooled furnace.
[0064] A portion (250 g) of the calcined alumina extrudate was impregnated with an aqueous solution of chromic acid (82.4 g), a sodium dichromate solution (12.4 g, 69% sodium dichromate dihydrate) and water (58.8 g) To first wet. The samples were dried and calcined in air at 750 degrees Cels...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com