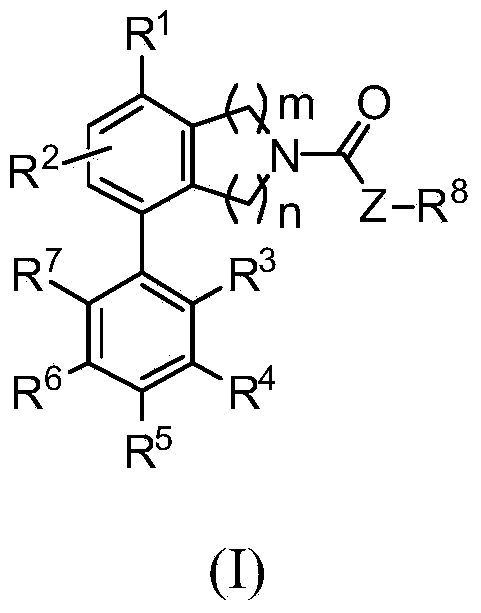
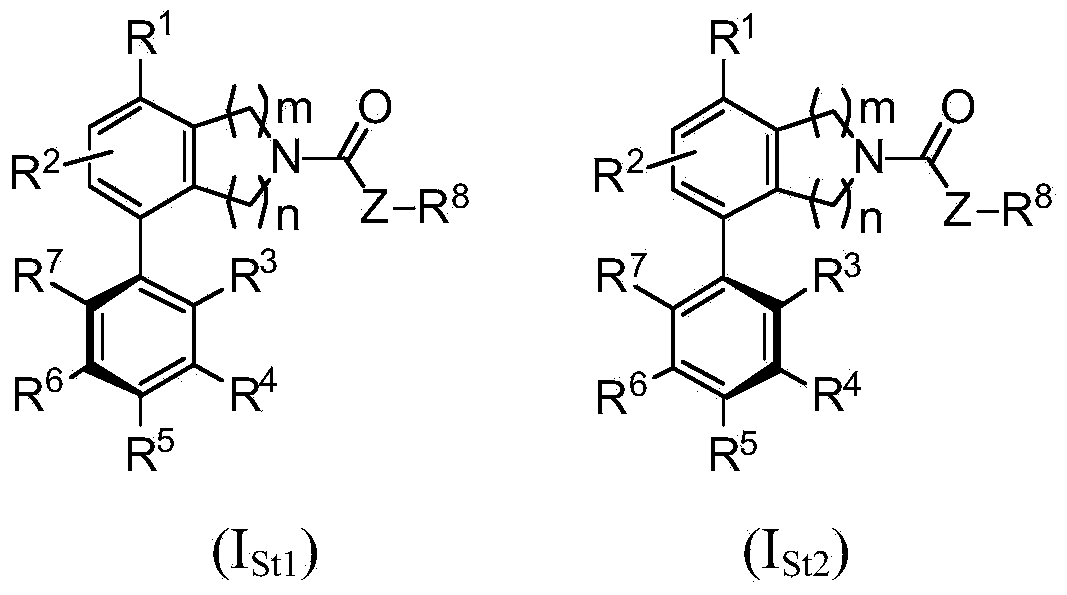
Heterocyclyl derivatives and their use as prostaglandin d2 receptor modulators
A halogen and compound technology, applied in the field of formula heterocyclic derivatives and as prostaglandin receptor modulators, can solve problems such as limited efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1165] The following examples illustrate the preparation of the compounds of the invention but do not limit their scope in any way. Whenever used in the experimental part, general structures 1, 2, 3, etc. refer to the respective structures described in the preceding general description of the preparation of compounds of formula (I) below.
[1166] General method for preparing compounds of formula (I):
[1167] Saponification
[1168] To (±)-(3-{5-fluoro-2-[trans-2-(4-fluoro-phenyl)-cyclopropanecarbonyl]-1,2,3,4-tetrahydro-isoquinoline-8 To a solution of ethyl}-4-methoxy-phenyl)-acetate in DMF (1 mL) was added 2M aqueous NaOH (1 mL). The mixture was stirred at r.t. for 18 hours. The solution was neutralized with formic acid (ca. 1 mL), purified by preparative HPLC (column: Atlantis, 18 x 50 mm, 10 μm, UV / MS, acidic conditions) and concentrated in vacuo to afford the desired acid as a white solid.
[1169] Listed in Table 1 below are examples of compounds of formula (I) prep...
example 169
[1232] Example 169: Benzyl 8-(5-carboxymethyl-2-methoxy-phenyl)-5-cyano-3,4-dihydro-1H-isoquinoline-2-carboxylate (C27H24N2O5, MW =456.17)
[1233] To an ice-cooled suspension of 5-cyano-8-hydroxy-3,4-dihydro-1H-isoquinoline-2-carboxylic acid benzyl ester (73 mg, 0.24 mmol, 1.0 eq.) in DCM (4.4 mL) Triethylamine (99 μL, 0.71 mmol, 3.0 eq.) and trifluoromethanesulfonic anhydride (61 μL, 0.355 mmol, 1.5 eq.) were sequentially added to the solution. The reaction mixture was stirred at 0 °C for 30 min and at r.t. for another 45 min. The mixture was diluted with DCM (10 mL) and washed with saturated NaHCO 3Aqueous (2x) washes. The organic layer was washed with MgSO 4 Dry, filter and concentrate in vacuo. To triflate, [4-methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl] - ethyl acetate (97mg, 0.24mmol, 1.0eq.) and sodium carbonate (100mg, 0.95mmol, 4.0eq.) in toluene / EtOH / water 20:4:1 (4.8mL) in N 2 To the mixture below was added tetrakis(triphenylphosphine)pal...
example 170
[1235] Example 170: Benzyl 8-(5-carboxymethyl-2-methoxy-phenyl)-3,4-dihydro-1H-isoquinoline-2-carboxylate (C26H25NO5, MW=431.17)
[1236] To an ice-cooled solution of benzyl 8-hydroxy-3,4-dihydro-1H-isoquinoline-2-carboxylate (113 mg, 0.40 mmol, 1.00 eq.) in DCM (1 mL) was added NEt in sequence 3 (0.17mL, 1.20mmol, 3.00eq.) and trifluoromethanesulfonic anhydride (0.10mL, 0.60mmol, 1.50eq.). The reaction mixture was stirred at 0 °C for 30 min and at r.t. for another 45 min. The mixture was diluted with DCM (50 mL) and washed with saturated NaHCO 3 Wash with aqueous solution (2 x 25 mL). The organic layer was washed with MgSO 4 Dry and concentrate in vacuo. To triflate, [4-methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl] - ethyl acetate (128mg, 0.40mmol, 1.00eq.) and sodium carbonate (170mg, 1.60mmol, 4.00eq.) in toluene / EtOH / water 20:4:1 (2.5mL) in N 2 To the lower mixture was added tetrakis(triphenylphosphine)palladium(0) (23mg, 0.02mmol, 0.05eq.). The m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com