Inhibition Of Viral Gene Expression
A virus and hepatitis virus technology, applied in gene therapy, antiviral agents, genetic engineering, etc., can solve the problems of enlarged synthesis reaction, low yield and difficulty of multi-step chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
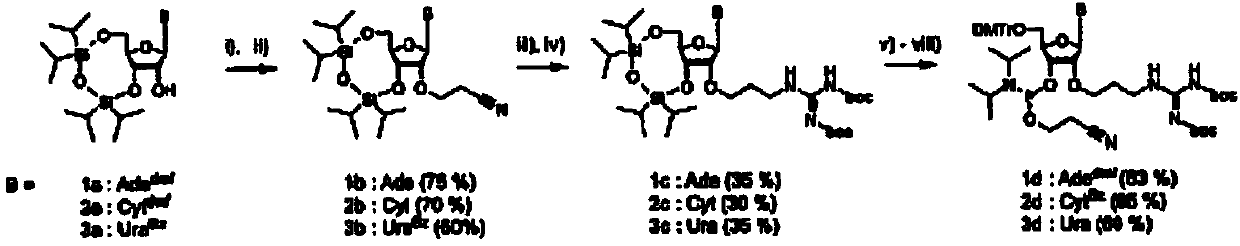
[0063] Example 1 Synthesis of four kinds of 2'-O-guanidinopropyl-nucleoside-phosphoramidites
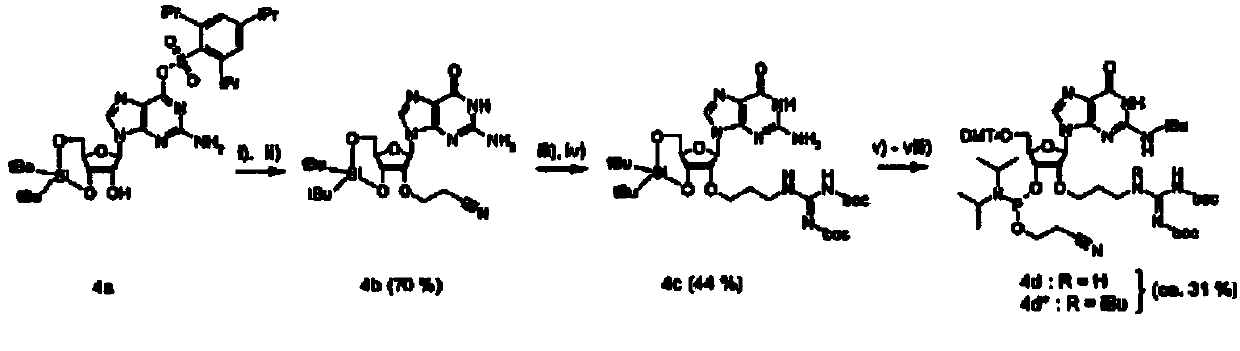
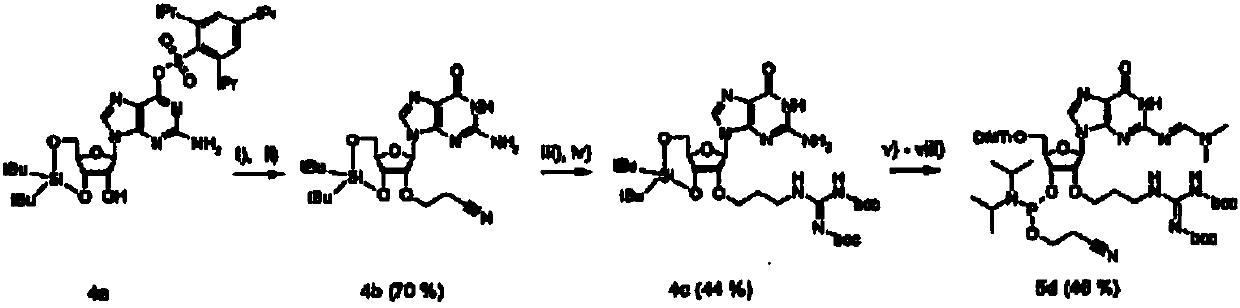
[0064] Each of the four 2'-O-guanidinopropyl-nucleoside-phosphoramidites was synthesized using essentially similar methods. figure 1 The synthesis of adenosine (A), cytidine (C) and uridine (U) derivatives is described. Separately in the diagram ( figure 2 )show. Each synthesis starts with the use of 1,1,3,3-tetraisopropyldisiloxane-1,3-diyl (TIPS) (for A, C and U) or di-tert-butylsilanediyl ( DTBS) (for guanosine) protects both the 5'- and 3'-hydroxyl groups. DTBS was chosen to protect G because this group has been reported to increase the selectivity of subsequent 2,4,6-triisopropylbenzenesulfonyl (TPS) protection at the O6-position of guanosine [21]. The exocyclic amino functions of A and C were protected with a dimethylaminomethylene group using standard conditions, and a benzoyl group was attached at the N3-position of U using a two-phase system reported by Sekine [22]. Th...
Embodiment 2
[0069] Example 2 Synthesis of 2'-O-guanidinopropyl adenosine phosphoramidite
[0070] 3′,5′-O-(tetraisopropyldisiloxane-1,3-diyl)-N6-dimethylaminomethyleneadenosine (1a) was synthesized as previously described [16].
[0071] N6-Dimethylaminomethylene-2′-O-cyanoethyl-3′,5′-O-(tetraisopropyldisiloxane-1,3-diyl)-adenosine (1e)
[0072] Freshly distilled acrylonitrile (6.7 mL, 102 mmol) and cesium carbonate (1.6 g, 4.9 mmol) were added to a solution of compound la (3.0 g, 5.31 mmol) in tert-butanol (25 mL). The mixture was stirred vigorously at room temperature for 3 h. The reaction mixture was filtered and the residue was washed with dichloromethane. The filtrate was evaporated and the residue was purified by column chromatography with ethyl acetate / methanol (99:1 - 95:5, v / v) yielding 3.28 g (87%) of product. 1H NMR (400MHz, DMSO-d6) δ [ppm] 8.90 (s, 1H, amidine-H), 8.34 (s, 1H, H2 or H8), 8.32 (s, 1H, H2 or H8), 6.02-6.01 ( m,1H,H1′),5.05-5.01(m,1H,H3′),4.64-4.62(m,1H,H2′),...
Embodiment 3
[0087] Example 3 Synthesis of 2'-O-guanidinopropylcytidine phosphoramidite
[0088] N4-Dimethylaminomethylene-3′,5′-O-(tetraisopropyldisiloxane-1,3-diyl)-cytidine (2a) according to the previously described reaction procedure [29 ]synthesis.
[0089] N4-Dimethylaminomethylene-2′-O-cyanoethyl-3′,5′-O-(tetraisopropyldisiloxane-1,3-diyl)-cytidine (2e)
[0090] Compound 2a (4 g, 7.39 mmol) was dissolved in acrylonitrile (8 mL, 122 mmol) and tert-butanol (35 mL). Cesium carbonate (1.8 g, 5.52 mmol) was added and the reaction mixture was stirred at room temperature for 2.5 h. The mixture was filtered through celite, and the residue was purified by column chromatography after evaporation of the solvent. Ethyl acetate was used initially as solvent, which was changed to ethyl acetate / methanol (9:1, v / v) after the non-polar mixture was passed through the column. The product was obtained in a yield of 3.78 g (86%). 1H NMR (400MHz, DMSO-d6) δ [ppm] 8.62 (s, 1H, N4 = CH-NMe2), 7.88 (d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com