Tetravinyl-based Gemini type amphiphilic compound as well as preparation method and application thereof
An amphiphilic compound, tetravinyl technology, applied in the field of Gemini-type amphiphilic compounds, can solve the problems of great difference in transfection efficiency and single function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The Gemini type amphiphilic compound based on tetravinyl shown in synthetic formula (I),
[0084]
[0085] R represents straight-chain alkyl or straight-chain monoalkenyl; In this embodiment, R represents C 8 、C 12 and C 18 straight chain alkyl, and (E)-9-octadecenyl as an example; R represents C 8 When it is a straight chain alkyl group, it is compound 1, and R represents C 12 When it is a straight chain alkyl group, it is compound 2, and R represents C 18 It is compound 3 when straight-chain alkyl, and compound 4 when R represents (E)-9-octadecenyl.
[0086] The synthetic route is:
[0087]
[0088] The preparation process of route 1 compounds 1, 2, 3 and 4: (i) zinc powder, titanium tetrachloride, tetrahydrofuran, -78 ° C to 60 ° C, overnight; (ii) potassium carbonate, potassium iodide, acetone, reflux, 48 hours ; (iii) n-butyllithium, THF, -78°C to room temperature; (iv) a: copper sulfate pentahydrate, sodium ascorbate, THF / water, argon, room temperature...
Embodiment 2
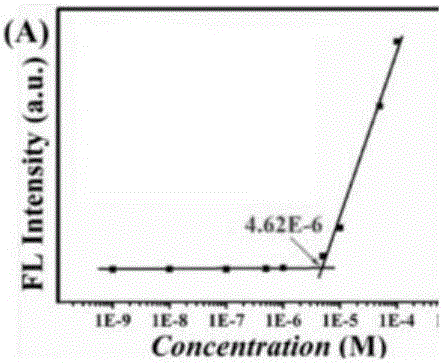
[0111] Compounds 1, 2, 3 and 4 were configured into aqueous solutions of different concentrations, and the fluorescence intensity of the aqueous solutions of compounds 1 to 4 was measured; the maximum value of the fluorescence intensity varied with the concentration (10 -9 -10 -3 M) change trend plotting, get Figure 1A ~ Figure 1D ,exist Figure 1A-1D In , the X-axis is the solution concentration, and the Y-axis is the fluorescence intensity.
[0112] Figure 1A-1D They are the determination charts of critical micelle concentration and micelle morphology of compounds 1-4, respectively. Figure 1A-1D It can be seen that the critical micelle concentrations of compounds 1 to 4 are all very low, and the critical micelle concentrations of compounds 1 to 4 are 4.62×10 - 6 M, 4.58×10 -6 M, 4.02×10 -6 M and 4.94×10 -6 M.
[0113] Take 2 μL of the aqueous solution of mixture 1 to 4 with a concentration of 5 μM, drop them on a 200-mesh copper grid, and dry them naturally. Fi...
Embodiment 3
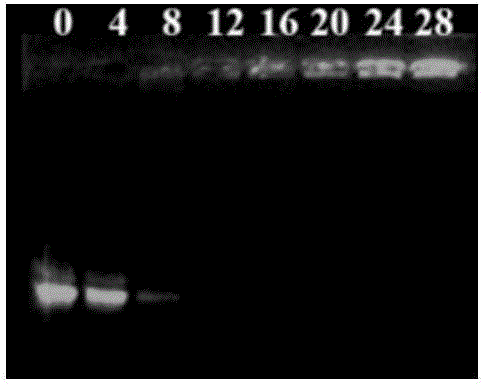
[0116] Solutions of compounds 1-4 with different concentrations were prepared respectively, and the solutions of compounds 1-4 with different concentrations and pUC18 plasmid DNA (9 μg / mL) were placed in a 37°C water bath for 1 hour, and the DNA agarose gel retardation experiment was carried out to obtain Gel retardation results of pUC18 DNA with different concentrations of compounds 1-4.
[0117] Figure 2A-2D Respectively, the results of the agarose gel retardation experiment of the pUC18 DNA of compounds 1-4 of the present invention; Figure 2A-2D The value marked above is the test concentration (μM); Figure 2A-2D It shows that compounds 1-4 can completely block the migration of DNA in agarose at lower concentrations; the lowest blocking concentrations of compounds 1-4 are: 16 μM, 16 μM, 12 μM and 30 μM, respectively.
[0118] It can be concluded from Example 2 that the tetravinyl-based Gemini amphiphilic compound prepared by the present invention has good self-assembly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com